Introduction
Traumatic vertebral artery injuries (TVAI) should be suspected clinically in the setting of cervical spine trauma with symptoms of neck pain and/or vertebrobasilar ischaemia. Blunt cerebrovascular injury (BCVI) is the umbrella term used for traumatic vascular injuries of the head and neck arteries; however, we have limited the scope of this article to imaging of TVAI.
The major reasoning for early detection of BCVI is to prevent the possibility of stroke, which can occur in 10-13% cases, resulting in potential permanent neurological deficits [1]. With early detection of BCVI, the incidence of stroke can be reduced to as low as 0.5% by initiation of appropriate treatment, which can include antithrombotic medications or vascular intervention versus untreated BCVI, in which the incidence of stroke can reach 21% [2].
The clinical scenarios for both BCVI and TVAI are similar. There have been several grading systems and screening criteria set up for detection of BCVI, including the Denver [3], Memphis [4], and Boston [5], criteria based on injury mechanism, injury pattern, and symptoms. The initial screening criteria were set up in the 1990s [6] and were further expanded by the Denver group in 2005 [3]. This modified Denver criteria is the most widely used criteria in practice [1]. More recent literature from 2011 proposed expanding screening criteria to detect asymptomatic early-stage BCVI in order to further reduce the risk of stroke [7]. Application of this expanded screening criteria has improved detection of BCVI from 2.3 to 2.9% [8] (Table 1 depicts the expanded screening criteria for detection of BCVI proposed in 2011 by Burlew et al. [7]).
Table 1
The 2011 expanded screening criteria for detecting blunt cerebrovascular injury (BCVI) (adapted from [7])
| Signs/symptoms of BCVI | Risk factors for BCVI |
|---|---|
| Arterial haemorrhage (from neck, nose, or mouth) | High-energy transfer mechanism associated with: |
| Cervical bruit (in younger than 50 years of age) | Facial fracture of LeFort II or III type |
| Expanding cervical haematoma | Mandible fracture |
| Focal neurologic defect: transient ischemic attack, hemiparesis, vertebrobasilar symptoms, Horner syndrome | Complex skull fracture/basilar skull fracture/occipital condyle fracture |
| Neurologic deficit inconsistent with head computed tomography | Closed head injury consistent with DAI and GCS < 6 |
| Stroke on computed tomography or magnetic resonance imaging | Cervical vertebral body or transverse foramen fracture, subluxation, or ligamentous injury at any level; any fracture at C1-C3 |
| Near hanging with anoxic brain injury | |
| Clothesline-type injury or seat belt abrasion with significant swelling, pain, or altered mental status* | |
| Traumatic brain injury with thoracic injuries* | |
| Scalp degloving* | |
| Thoracic vascular injuries* | |
| Blunt cardiac rupture* |
However, a balance has to be struck between excess imaging and not missing out on asymptomatic vascular injury. Use of expanded screening criteria or ordering CT angiography (CTA) for all cervical spine fractures can lead to a higher false negative rate. The decision to use either conservative classic screening criteria or more expanded screening criteria remains controversial and an area of ongoing research. However, there remains no doubt that angiographic imaging is mandated for detecting vascular injury in cases of complex cervical spine fractures, subluxations, fractures involving facet joints, or the transverse foramina (covered under the modified Denver criteria), which tend to have a high incidence of concomitant vascular injury and higher yield of vascular injury cases [9]. However, the caveat remains that utilising limited screening criteria can miss up to 20% of cases. In conclusion, the decision regarding the optimal screening criteria will have to be made by an institution based on its available resources and local standards of care.
Imaging modalities for detection of traumatic vertebral artery injuries
Digital subtraction angiography (DSA) has historically been considered the gold standard for diagnosis of vascular injury in the head and neck. But DSA is an invasive test, which may not be readily available at smaller hospitals. An invasive test also makes it a less favourable option to be used as a screening tool. DSA is also limited to the evaluation of the vessel lumen and can miss out on detecting cases with non-stenotic intramural haematoma [10]. The role of DSA for diagnosis now would be limited to evaluating high-risk patients who have equivocal or negative results on CTA [11].
Now with improvements in CT technology, CTA has proven to be the initial screening test of choice in head and neck vascular imaging. CTA has recently shown remarkably improved detection of BCVI, especially with 64-slice and higher CT scanners [12]. In one study, CTA demonstrated a high sensitivity of close to 98% and specificity close to 100%, when compared to DSA [13]. However, achieving these high numbers requires that the radiologists are able to sustain the quality of reading by being more familiar with the spectrum of imaging in BCVI and TVAI, which is the aim and scope of this article.
Doppler Duplex US has a very limited problem-solving role. It cannot be used for screening due to its poor sensitivity because large portions of the vertebral arteries are not accessible for sonographic detection. Hence, its sensitivity for TVAI is even lower than for detection of carotid injuries [14]. The advantages of doppler US, however, are its safety, availability, and ability to detect direction of flow in the vertebral arteries.
Magnetic resonance angiography (MRA) plays a complementary role when combined with MRI for concomitant detection of strokes with MRI and vascular injury with MRA. However, when judged by its ability to detect vascular injury, it is less sensitive compared to either CTA or DSA. Studies have found low sensitivities for MRA for detection of TVAI in the range of 47-60% [4].
We present a brief overview of normal and variant anatomy of the vertebral arteries. We follow that with a pictorial review of the imaging spectrum of TVAI including grading of the injury along with the mimics and pitfalls to be aware of.
Anatomy
Normal anatomy
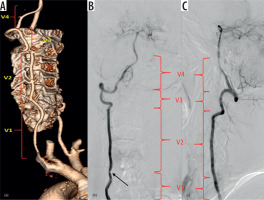
The vertebral artery (VA), in most people, are paired structures arising from the first part of the subclavian arteries. It then ascends posterior to internal carotid artery, obliquely superiorly and medially towards the cervical spine. Either at the C6 or C7 level, it enters the foramen transversarium/transverse foramen, which is an aperture within the transverse processes of the cervical vertebrae. It ascends through the foramen transversaria till C2. The transverse foramina contain VA, vertebral veins, and sympathetic plexus. From C2, the VA sweeps laterally to pass through the transverse foramen of C1 and passes superiorly to pierce the dura and arachnoid to extend intracranially and then joins the contralateral VA to form the basilar artery. Anatomical landmarks have been utilised to divide the vertebral artery into segments – the V1 (extraosseous segment) from origin to entry into its caudal most transverse foramen, V2 (foraminal segment or pars transversaria) – typically from C6 to C2 within the transverse foramina, V3 (extraspinal segment or atlas loop) from C2 to dura at the skull base, and V4 (intradural intracranial segment) from dura to confluence (Figure 1).
Figure 1
Three-dimensional (3D), volume-rendered (A), and digital subtraction angiography images (B-C) show the vertebral artery segments. V1 – between vertebral artery origin and its entry into the transverse foramen of the C6 vertebra, V2 – foraminal course between the transverse processes of C6 to C2, V3 – atlas loop region, and V4 – intracranial segment. Note there is luminal narrowing in V1 segment in image (B) (black arrow)
Variant anatomy
The VA may arise directly from the brachiocephalic arteries or directly from the aortic arch instead of the subclavian artery. The VA may be asymmetric in size with one vessel being dominant. The left VA is more commonly dominant. The finding of VA hypoplasia, although a developmental variant, has been reported to have a higher incidence of VA dissection and posterior circulation stroke [15]. It can sometimes be difficult to conclude whether smaller size of the VA is due to developmental hypoplasia or due to acquired stenosis. Hypoplasia is usually a diffuse finding and the entire VA is diffusely small calibre. However, there may be, on occasions, an isolated narrow V4 segment after the origin of the posterior inferior cerebellar artery or even the V4 segment terminating as the posterior inferior cerebellar artery [16]. These should not be mistaken for stenosis or TVAI. A useful clue for developmental hypoplasia is that the size of the transverse foramen in also small with a developmentally small VA. Stenoses tend to occur segmentally whereas hypoplasia is a diffuse finding. Duplication and fenestration of the vertebral arteries and of the vertebra-basilar junction is also a rare variant [17,18]. This should be identified as a variant and not mistaken for the two-vessel sign of TVAI.
Mechanism and grades of traumatic vertebral artery injuries
The mechanism of TVAI usually involves both pre-existing intrinsic susceptibility and a precipitating event. Although a normal VA can undergo injury from major trauma, minor trauma can also cause TVAI when there is an underlying arteriopathy, which may be congenital or genetic, like connective tissue disorders, fibromuscular dysplasia, or vessel wall weakening caused by infection or inflammation of the vessel wall. Pathological evaluation of cases of spontaneous cervical artery dissections have found changes of Ehlers Danlos syndrome and Marfan syndrome in the vessel wall [19]. This, superimposed with a minor or major trauma, leads to intimal tears, which are the subtlest form of injury. These tears can be seen as thin intimal flaps on imaging in the vessel lumen. This dissection can progress further when luminal blood enters through the intimal defect and extravasates in the vessel wall creating a false lumen. The false lumen can extend for a variable length and can remain patent or more frequently thrombose due to slower flow. More severe injury can manifest when the vessel wall ruptures, and a pseudoaneurysm may be formed if the rent in the vessel wall is small. In most severe forms of injury, the vessel may become occluded or transacted, which is usually fatal [20-22].
On review of the literature, the predominant aetiology was found to be high-velocity motor vehicle-related injuries; however, other cause like a fall, sporting accident, chiropractic manipulation, and hanging, although less common, have been described [9]. Distractive flexion injury is the main mechanism for TVAI, instead of the hyperextension injury as previously thought [23,24].
Location of TVAI: Most TVAI are located in the V2 or V3 segment [25,26]. The V2 segment is the most commonly affected in adult TVAI. In infants and children, the V3 and upper V2 segment are more commonly affected [27].
The grading of TVAI severity proposed by the Denver group is the most widely used and is depicted in Table 2 [9]. We subsequently present examples of these grades in the imaging spectrum that follows.
Table 2
Denver grading of severity of blunt cerebrovascular injury (BCVI) (from [3])
Imaging spectrum
Computed tomography angiography
Technique: CT angiography (CTA) of head and neck, when performed with modern 64-slice or higher CT scanner, provides thin-slice (1 mm or less), high-resolution imaging of the arterial lumen and the wall of the VA. Bolus tracking of the contrast is incorporated in the protocol in order to obtain optimal phase of maximum opacification of the arteries. Modern CT scanners are faster at scanning as well limiting late contrast bolus and artefacts from thicker slices associated with older CT scanners. Generation of multiplanar coronal and sagittal reconstructions, MIP (maximum intensity projection), and VR (volume rendered) imaging are among the common protocols for CT angiographic imaging, and these multiple views and projections help to establish a more confident assessment of the anatomy and pathology.
CTA Findings of TVAI: The findings on CTA will vary as per the grade of injury. The commonly encountered imaging findings of TVAI on CTA are smooth tapered luminal narrowing, concentric or focal luminal narrowing of the VA (Figure 2A-C), or total occlusion (Figure 3), with these findings seen in more than 50% cases. Other findings seen are, double lumen (Figure 4A), intimal flap (Figure 4B-G), dilatation of artery (Figure 4F-H), beaded appearance of artery or string of pearls appearance (Figure 4I-K) or stenosis of lumen. The most definite sign is double lumen or intimal flap, which is not frequently seen (in around 22% cases), and the most common imaging characteristic is nonspecific luminal stenosis (seen in more than 50% cases) [28]. CTA can show contrast extravasation or pseudoaneurysm formation, which are signs of more severe injury (Figure 5).
Figure 2
Axial non-contrast time-of-flight (TOF) magnetic resonance angiography (MRA) image (A) and reformatted 3D maximum intensity projection image (B) showing smooth tapered narrowing of the left vertebral artery consistent with grade II traumatic vertebral artery injury (TVAI) (white arrow). Another patient’s axial TOF MRA image (C) showing irregularity of lumen of left vertebral artery suggesting focal dissection (grade I TVAI) and the same patient’s axial computed tomography (D), diffusion weighted image (E), and ADC map (F) of the brain showing acute left cerebellar infarct
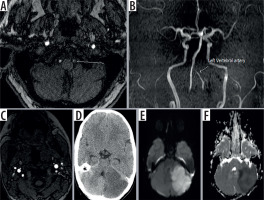
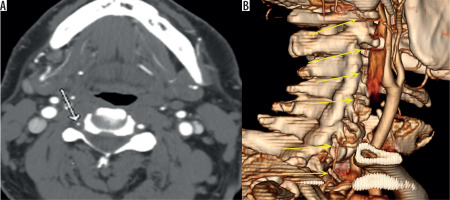
Figure 3
Axial contiguous computed tomography images (A-C) showing total occlusion of the right vertebral artery. The reformatted image (D) shows opacification of the upper portion of the vertebral artery, probably from retrograde filling of the lumen (white arrow). Three-dimensional volume-rendered image (E) showing occlusion of the V1 segment of the vertebral artery (yellow arrow)
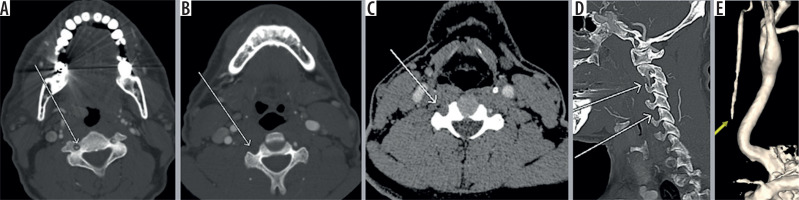
Figure 4
Axial computed tomography angiography (CTA) image (A) showing double lumen in right vertebral artery s/o dissection (grade II traumatic vertebral artery injury [TVAI]). Multiplanar reformatted image (B-C) showing the intimal flap (grade II). Contiguous slices of axial CTA images (D-E) of another patient showing intimal flap in left vertebral artery (green arrow). Axial CTA image (F) showing increase in luminal diameter of the left vertebral artery (green arrow). The reformatted image (G) in same patient showing the dilatation (white arrow) of the artery with intimal flap in the lumen (red arrow) and three-dimensional (3D) volume-rendered image (H) showing abrupt expansion of the artery (white arrow). Another patient’s coronal magnetic resonance angiography (MRA) image (I) showing beaded narrowing of the left vertebral artery (red arrow) and the reformatted image (J) showing the irregular filling of the left vertebral artery (white arrow). The three-dimensional (3D) volume-rendered image (K) in the same patient showing beaded appearance of left vertebral artery “string of pearls appearance” (green arrow). Comparative normal appearance of contralateral vertebral artery on the three-dimensional (3D) volume-rendered image (L)
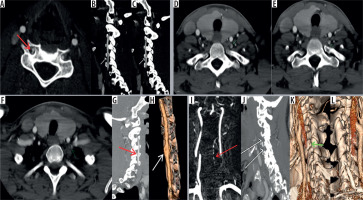
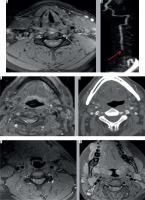
Figure 5
Axial computed tomography angiography (CTA) image (A) showing contrast outpouching from the V3 segment of the left vertebral artery (white arrow). The reformatted image (B) shows the fracture through the transverse foramen (white arrow). The axial magnetic resonance image (C) shows the small signal outpouching from the same site as was depicted on CTA (white arrow) and the three-dimensional (3D) volume-rendered image (D) showing the pseudoaneurysm clearly. CTA images (E, F) showing left pseudoaneurysm (yellow arrow) with fractures through the foramen transversarium (red arrows); also note incidental detection of a hypervascular right glomus tumour adjacent to cervical internal carotid artery (black arrow)
CTA Pitfalls: The CTA images may be affected by poor bolus timing, bone, dental, or metallic artefacts in the spine or from patient’s motion, swallowing, or pulsation artefacts, which limit image interpretation. Poor bolus may show contrast in arteries and veins simultaneously, giving the appearance of two lumens. Tortuosity of the vessel may also give the false appearance of two lumens. Atherosclerotic plaque or ulcerated plaques may have an appearance of intimal flap with contrast in vessel the lumen and in the ulceration outside the expected location of the arterial wall, and lead to false positive interpretation of TVAI. This is an issue with evaluation in older patients who have pre-existing atherosclerotic changes in their vessels. Arterial wall thickening or diminished flow due to severe stenosis may cause false positive interpretation for TVAI [29]. Developmentally hypoplastic VA can mimic long segment arterial injury, but usually can be ascertained by a smaller ipsilateral transverse foraminal diameter (Figure 6). Another limitation is that CTA is not a dynamic study, so it is difficult to interpret if there is retrograde filling of the affected vertebral artery or reversal of flow. Refer to Table 3 for a summary of the TVAI CTA findings and pitfalls.
Table 3
Findings and pitfalls of traumatic vertebral artery injury on computed tomography angiography
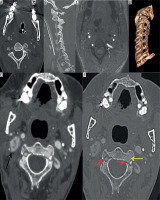
Figure 6
Axial computed tomography angiography image (A) showing asymmetry of lumen of the right vertebral artery. Three-dimensional (3D) volumerendered image (B) shows that the whole of the right vertebral artery is hypoplastic (yellow arrows)
Care should be taken while calling in a TVAI because there could be other medical conditions that can lead to similar findings of occlusion or stenosis. The patient’s clinical history should be kept in mind. The most common mimickers are fibromuscular dysplasia, atherosclerosis, and post-radiation treatment changes.
Magnetic resonance angiography and magnetic resonance imaging
Technique: TVAI can be assessed with MRA either with or without contrast. The ability to image without contrast is especially helpful in patients with contraindications to iodinated contrast material. The imaging protocol for non-contrast MRA includes either 2D or 3D time-of-flight imaging, which detects the velocity of flowing protons. Another technique is phase contrast imaging, which can provide quantitative estimation of the flow velocity. T1 images of the neck with fat saturation are also performed as part of standard protocol because the blood in the false lumen – the intramural haematoma – appears hyperintense in the subacute phase (Figure 7). Usage of gadolinium contrast is optional, but it does improve the imaging of the lumen of the arteries secondary to opacification by T1 relaxation effects of gadolinium. MRI of the brain can be performed at the same time as the MRA. The MRI, especially diffusion sequence, can help detect the presence of acute infarction, for which it is exquisitely sensitive and specific when compared to CT. Other factors that affect the imaging characteristics are the field strength of the scanner, patient body habitus, the signal intensity from the surrounding structures, and the imaging sequence protocol used [30,31].
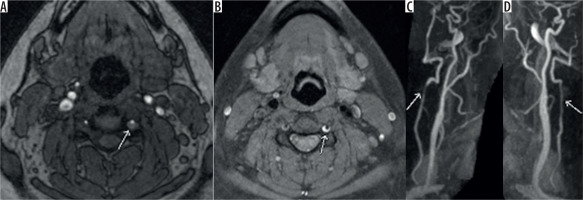
Figure 7
Axial time-of-flight magnetic resonance angiography image (A) showing intramural haematoma as seen by abnormal signal surrounding and narrowing the left vertebral artery flow signal (white arrow). T1 fat-saturated image (B) showing the T1 hyperintense signal consistent with intramural haematoma (white arrow) and the reformatted images showing luminal narrowing (C, D) (white arrow)
Magnetic resonance angiography findings of traumatic vertebral artery injuries
The intramural haematoma in the false lumen after dissection is hyperintense on T1-weighted images in subacute stages between seven days and two months and is detected on the fat-saturated imaging. Subacute haematoma is seen as crescentic hyperintense areas around an eccentric flow void [30,32]. However, it is isointense in acute or chronic stages (within six months). When the haematoma is isointense, as in acute or chronic dissections, it becomes tough to detect. Other findings seen on MRA are loss of flow void or occlusion (Figure 8A-B), similar to what can be seen on CTA. The 3D TOF technique is better than the 2D TOF in regard to spatial resolution, but it increases the scan time. Contrast MRA has greater ability to evaluate the lumen, especially for subtle luminal irregularities and stenosis [32,33].
Figure 8
Axial T1 fat-saturated magnetic resonance (MR) image (A) showing loss of flow void in left vertebral artery and abnormal hyperintense signal around it s/o dissection with intramural haemorrhage (white arrow). Reformatted time-of-flight magnetic resonance angiography image (B) shows complete occlusion of the left vertebral artery (red arrow) with distal reconstitution. Another patient with axial T1 fat-saturated MR image (C) showing hyperintense signal in right vertebral artery with loss of flow void, and the computed tomography angiography (D) performed the same day showed contrast filling of the right vertebral artery; thus, it was concluded that the abnormal signal is secondary to the flow artefact. Other patient axial MR images (E, F) showing venous contamination producing hyperintense signal along the periphery of bilateral vertebral arteries. Note asymmetry in the size of the vertebral arteries, with left being dominant
MRA Pitfalls: The false positives on MRA are due to turbulent flow producing hyperintense signal in the periphery with central flow void simulating stenosis or intramural haematoma (Figure 8C-D). Additionally, periarterial structures like fat or veins can simulate intramural haematoma or pseudoaneurysm (Figure 8E-F). These are more confounding when there is developmental asymmetry in the size of the VA (Figure 8F). Comparison with other sequences, fat saturation, or CT images may be helpful in such cases [29,34]. Entry slice phenomenon causing a bright signal in the artery at the extreme of the imaging volume due to entry of unsaturated spin can simulate an intramural abnormal signal or the absence of an expected flow void simulating dissection. Also, the flow-related enhancement in the veins adjacent to VA can simulate intramural haematoma or dissection on unenhanced MRA (TOF). These can be overcome by the application of saturation pulses cranially and caudally to the imaging volume [29,35]. V1 segment VA dissection may be missed with V1 inadvertently not included in the image volume, and small dissection may be missed due to gaps in image slabs [31]. In early or acute stages, the intramural haematoma may be isointense on T1-weighted images, in such cases other signs may be helpful, like luminal narrowing or pseudoaneurysm formation. In subacute stages, the bright intramural haematoma may be masked by the background bright signal from fat or indistinguishable from the flow-related enhancement on MRA. The bright signal from an occluded vessel may obscure the bright intramural haematoma; however, this can be resolved by contrast-enhanced MRA with subtraction of unenhanced images [29]. Asymmetry in the size of the VA may be mistaken for dissection in a smaller lumen artery (Figure 8F). Careful observation of the periarterial bright signal on TOF, only seen in dissection rather than normal artery, should be performed [34]. Comparing the luminal diameter on the VA above and below the site of injury can help in calling in or ruling out normal asymmetry with confidence. In contrast-enhanced MRA there might be segmental blurring of the signal in VA due to rapid circulation times, especially in young patients, called the feathering artefact, and it may simulate small stenosis. This can be overcome by using an earlier trigger time with elliptical centric contrast enhanced MRA [33]. MR is also limited in the detection of pseudoaneurysms because they are mostly seen at the skull base with abnormal flow or signal within and may mimic a mass lesion [34,36]. Refer to Table 4 for a summary of the TVAI MRA findings and pitfalls.
Table 4
Findings and pitfalls of traumatic vertebral artery injury on magnetic resonance angiography (MRA)
Digital subtraction angiography
DSA, although traditionally considered a gold standard, is limited by lack of ability to assess the arterial wall configuration and thickness. The common signs seen on DSA are: complete occlusion of lumen (Figure 9), “string sign”, and “string and pearl sign”, which refers to long tapered eccentric irregular stenosis without or with distal dilatation. Pathognomonic signs, such as intimal flap and or double lumen, are rarely observed [37]. When there is a tapered occlusion, it is referred to as the “Flame Sign” [38]. The major advantage of DSA is that it is a dynamic acquisition. Hence it does not suffer as much from contrast kinetics and bolus timing as CTA. Hence, DSA is sometimes performed to supplement evaluation in indeterminate cases, assess the collateral circulation, or rarely as part of endovascular repair. If there is good collateral circulation, patients usually do well with conservative management (Figure 10).
Figure 9
Digital subtraction angiography images (A, B) showing complete occlusion of right vertebral artery without reconstitution by collaterals. Timeof- flight magnetic resonance angiography image (C) showing absence of flow signal in the right vertebral artery (yellow arrow). Reformatted image (D) confirming the same with absent signal of right vertebral artery
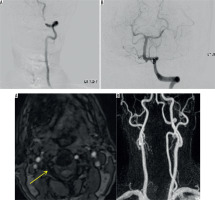
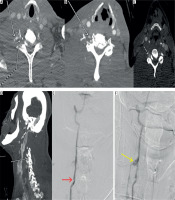
Figure 10
Computed tomography angiography (CTA) image (A) showing the factures through the transverse foramen (white solid arrow) with irregularity of the right vertebral artery (white arrow with dotted line). CTA Image (B, C) showing mild dilatation of the right vertebral artery with intraluminal flap (grade II injury) (white arrow). Follow-up reformatted image (D) showing pseudoaneurysm formation in right vertebral artery (white arrow). Angiographic images (E) demonstrating the dissection with intimal flap (red arrow) and pseudoaneurysm (yellow arrow) (F). Patient had good collateral circulation without enlargement of pseudoaneurysm and so was managed conservatively
Doppler duplex ultrasound
US doppler duplex imaging has limited sensitivity in detecting mainly extracranial VA dissection. Distal V2 and V3 segments cannot be adequately evaluated due to a poor acoustic window. However, there are some features that are beneficial, like rapid mobility, cost-effectiveness, and non-invasiveness, but it is not sensitive enough to exclude intimal tears [39]. Hence, it is not suited to initial evaluation of a trauma patient when injury needs to be ruled out. However, it can be useful as a problem-solving tool. The ability to easily detect direction of flow in the vertebral artery is also helpful when compared to CTA and MRA. Spectral imaging with peak systolic velocity values and waveform morphology helps estimate the presence of stenosis. If TVAI can be visualised accurately, then it has a role for follow-up of vertebral artery dissection [40].
Management and treatment
TVAIs are usually managed conservatively with either anticoagulation or antiplatelet therapy. The used anticoagulant medications include warfarin and heparin. Used antiplatelet agents include aspirin, clopidogrel, and dipyridamole. The optimal regimen with respect to agent, duration of treatment, or end point of therapy is not known. Often the associated injuries limit antithrombotic therapy. In such cases, endovascular interventions are another therapeutic option [41]. Grade I and II injuries are managed conservatively with medical management. Anticoagulation, typically with heparin, acutely followed by three months of warfarin was the traditional management. In 2015, the Cervical Artery Dissection in Stroke Study trial (CADISS) suggested no difference in efficacy of antiplatelet and antithrombotic agents in preventing stroke and deaths in patients with symptomatic vertebral artery dissection [42]. This may encourage wider use of antiplatelet agents over anticoagulation agents considering the better safety profile of the former with respect to haemorrhagic complications. For grade III injuries with pseudoaneurysm, the initial treatment again is antithrombotic therapy. However, if the size of the pseudoaneurysm reaches from 1-1.5 cm or is symptomatic then endovascular stenting or coiling may be performed [41,43]. For grade IV injuries with arterial thrombosis, the initial treatment is with antithrombotic therapy, and treatment outcomes with endovascular therapy are not known yet. Grade V injuries are mostly fatal, and surgical exploration is recommended if possible.
Conclusions
Imaging with CTA is the initial test of choice in patients with trauma, who meet the screening criteria for the possibility of cervical vascular injury. MRA, DSA, and Doppler duplex US have complementary secondary roles in imaging. Early detection is key because initiation of therapy can dramatically reduce the incidence of associated strokes. Familiarity with the key findings of TVAI, anatomic variations, mimics, and pitfalls depicted and summarised in this review should assist the readers in more accurate estimation of TVAI.


