Introduction
Glucocorticoids (GS) have been used in the treatment of nephrotic syndrome (NS) since the 1950s [1]. They were found effective in inducing remission in approximately 90% of affected children and led to a substantial reduction in mortality (from 40% to less than 1%). On the other hand, GS carry the risk of complications such as arterial hypertension, diabetes, cataract, glaucoma, obesity, immunodeficiency, osteoporosis, and growth deficiency [2]. Despite many years of experience, there is still no established unambiguous treatment regimen of first bout and relapses of NS. Studies are aimed at reducing the number of NS relapses and minimizing GS doses and side effects. Both duration of the treatment and GS initial dose are matters of discussion. Two-, 3- and 6-month regimens have been used with conflicting data on the optimal treatment span [3, 4], though recent studies [5, 6] and guidelines [7, 8]suggest short treatment duration in the majority of affected children.
Also, the applied prednisone dose is a question of debate. To date, the dose proposed by the International Study of Kidney Diseases in Children (ISKDC) (60 mg/m2/24 h) is considered most effective [9]. Worldwide, a dose of prednisone converted into kg, i.e. 2 mg/kg (maximum 60 mg/24 h), is also used [9]. Prednisone doses calculated per kilogram of body weight compared to those calculated based on the body surface area are significantly lower in children with a body weight of < 30 kg [10]. In 2011 Saadeh et al. observed that prednisone underdosing resulting from dosing prescribed according to weight increases the likelihood of frequently relapsing course of NS [11]. Thus, both Kidney Disease Initiative Global Outcome (KDIGO) and the Polish Society for Paediatric Nephrology recommend initial treatment 60 mg/m2/24 h (not exceeding 60 mg/24 h) in the first month, 40 mg/m2/48 h in the following month with a 1-4 month further tapering period [2, 12]. In our department we used a weight-based regimen until 2013, then we adapted our treatment protocol strictly according to KDIGO recommendations.
The aim of the study was to compare the first year of the disease in children with NS treated according these two regimens: a weight-based schedule (i.e., 2 mg/kg/24 h in the first month, 2 mg/kg/48 h in the second month, with gradual dose tapering during the following 4 months), and a body surface area (BSA)-based schedule (i.e. 60 mg/m2/24 h in the first month, 40 mg/m2/48 h in the second month, with gradual dose tapering during the following 4 months).
Material and methods
The study group included 40 children (28 boys and 12 girls) aged from 1.64 to 11.56, mean 4.13 ±2.34 years, with the first bout of steroid-sensitive (SS) idiopathic nephrotic syndrome (INS). The patients were divided into two 20-children groups depending on the regimen of treatment.
Data in group I were collected retrospectively, based on medical records from 2010-2013. Group I consisted of 20 children aged from 1.64 to 4.98 years. In this group treatment of the first INS bout was as follows: prednisone 2 mg/kg/day (not exceeding 60 mg/day) for 1 month, 2 mg/kg/48 hours for 1 month, then reduced gradually for another 4 months. In the treatment of relapses, the same regimen was used. Recurrences of non-nephrotic proteinuria were treated with a daily prednisone in the same dose as previously used on alternate days until the date of resolution of proteinuria + 7 days, then the reduction of steroids continued. In this group, a patient weighing 30 kg with BSA 1 m2 received 4437.5 mg, i.e., 147.92 mg/kg of prednisone as treatment of the first INS bout.
Data in group II were collected prospectively in the years 2014-2016. The group consisted of 20 children aged from 1.77 to 11.56 years. The following regimen of treatment of the first bout of INS was used: prednisone 60 mg/m2/day (not exceeding 60 mg/day) for 1 month, 40 mg/m2/48 hours for 1 month, with gradual reduction of the dose during the following 4 months. In the treatment of relapses of INS prednisone was used at a dose of: 60 mg/m2/day (not exceeding 60 mg) until the day of resolution of proteinuria + 3 days, then 40 mg/m2/48 hours for 4 weeks with further reduction during the following 4 months. In the recurrences of non-nephrotic proteinuria, a daily dose of the previously used prednisone dose every 48 hours was used until the day of resolution of proteinuria + 3 days, then the reduction continued. In this group, a patient weighing 30 kg with BSA 1 m2 received 3620 mg, i.e., 120.67 mg/kg of prednisone as treatment of the first INS bout.
Patients from both groups were observed during the first year of the disease.
The diagnosis of NS was based on proteinuria > 50 mg/kg/24 h, hypoalbuminemia (≤ 2.5 g/dl), hyperlipidemia, and edema [9, 10]. Idiopathic nephrotic syndrome was defined based on exclusion of the following features in a child with NS: age of presentation < 1 year and > 12 years, constant erythrocyturia or gross hematuria, arterial hypertension (at presentation), hypocomplementemia, persistent impairment of renal function, and extrarenal manifestations suggesting systemic disease (e.g., arthritis, oral ulcerations). Steroid-sensitiveness was defined as entering remission within 8 weeks of prednisone treatment of INS. Time to remission was defined as the first day of a 3-day period of absent proteinuria in urinalysis in treatment of the first INS bout [2, 12].
Relapse of INS was recognized in case of recurrence of nephrotic range proteinuria (> 50 mg/kg/24 h) usually with hypoalbuminemia ≤ 2.5 g/dl. Recurrence of non-nephrotic proteinuria was defined as proteinuria < 50 mg/kg/day without a drop in serum albumin ≤ 2.5 g/dl. They were together analyzed as relapses of proteinuria (n).
The following parameters were analyzed in the studied children: age at presentation of first INS bout (years), sex, time to remission (days), total annual dose of prednisone (mg/kg/year), number of days without prednisone in the first year (days), number of relapsing patients (n), number of relapses of proteinuria (n), and presence of the following side effects of prednisone treatment: arterial hypertension, glaucoma, cataract, and disturbances of glucose metabolism. In addition, in all patients prior to the onset of prednisone treatment, and then after 12 months height (cm), body weight (initial weight was assessed after resolution of edema) (kg), and body mass index (BMI; kg/m2) were evaluated. All anthropometric variables were compared with Polish charts by Palczewska and Niedźwiecka and expressed as Z-scores [13]. Overweight was defined as a BMI Z-score > 1 and obesity as BMI > 2 according to World Health Organization (WHO) recommendations.
The results obtained were statistically analyzed using Dell Statistica 13.0 PL (TIBCO Software Inc., Palo Alto, CA, USA). Normality of data sets was established with the Shapiro-Wilk test. All data were presented as mean ±standard deviation and interquartile range. The differences between normally distributed data were evaluated using Student’s t-test, and between non-normally distributed data using the Mann-Whitney U test and Wilcoxon test. The number of patients in both groups was compared using Fisher’s exact test. The relations between quantitative variables were analyzed using Pearson correlation or Spearman’s rank correlation, when appropriate. Kaplan-Meier analysis and the log-rank test were used to compare one-year risk of INS relapse in two groups. A p-value < 0.05 was considered statistically significant.
Results
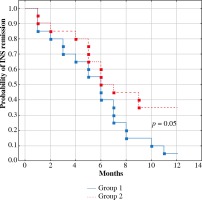
The clinical data and course of INS during the first year of follow-up in both groups were shown in Table 1. Patients in the first group (treated in the period 2010-2013) were significantly younger compared to patients in the second group (treated in the period 2014-2016). There was no difference between the groups in time to relapse or in number of relapses per year, but the second group was characterized by a lower number of relapsing patients, a lower total annual prednisone dose and a non-significantly higher number of days without prednisone. The log-rank test revealed higher probability of INS relapse in one-year observation in the first group (p = 0.05) (Fig. 1).
Table 1
Clinical data and course of idiopathic nephrotic syndrome in studied children
In group I arterial hypertension appeared soon after treatment initiation in one child and resolved after 5 months. In addition, arterial hypertension was observed in 3 other children from this group at 12 months of follow-up. In group II, arterial hypertension started in 4 children at the beginning of the treatment; after 12 months it was still present in one child and appeared in another child. There was no significant difference in number of hypertensive patients between the groups (4/20 vs. 5/20, p = 1.000). Ocular complications in the form of cataract or glaucoma were not found in any patients. Also, renal function was normal in all children at the beginning of the treatment and after 12 months. No glucose metabolism disturbances were observed in any of the analyzed children.
Anthropometric data in children with idiopathic nephrotic syndrome are shown in Table 2. Patients from group II were taller than patients from group I at the beginning of observation and after 12 years. The groups did not differ significantly in any other analyzed anthropometric variables. Before initiation of the steroid treatment there were 3 overweight and 3 obese children in group I, and one overweight and one obese child in the second group. After a year, these numbers increased to 5 overweight and 6 obese children in group I, and 5 overweight and 1 obese patient in the second group.
Table 2
Anthropometric data in children with idiopathic nephrotic syndrome
In the first group total annual prednisone dose (mg/kg/year) correlated negatively with annual change in height Z-score (r = –0.520, p = 0.018) and positively with annual change in BMI Z-score (r = 0.543, p = 0.013). Also, number of days without prednisone correlated negatively with BMI after 12 months (r = –0.570, p = 0.009), BMI Z-score after 12 months (r = –0.57, p = 0.011), increase in BMI (r = –0.617, p = 0.004), and increase in BMI Z-score (r = –0.636, p = 0.003). Total number of proteinuria relapses correlated positively with increase in BMI (r = 0.456, p = 0.043) and BMI Z-score (r = 0.387, p = 0.014). In the second group total annual prednisone dose (mg/kg/year) correlated negatively with annual change in height Z-score (r = –0.524, p = 0.018) and positively with annual change in BMI Z-score (r = 0.617, p = 0.004). Total number of proteinuria relapses correlated positively with weight Z-score after 12 months and negatively with annual increase in height Z-score (r = –0.597, p = 0.005).
Discussion
Our study focused on differences in clinical outcome between children with INS treated with two regimens of prednisone dosage. Analysis of our results revealed that the children treated with prednisone dosed for body surface area were found to be significantly less exposed to glucocorticoids. Nevertheless, this regimen did not influence the number of INS relapses or increase in body weight or BMI. Of note, in the weight-based regimen the second-month dose was substantially higher compared to the BSA-based regimen, which might have influenced the final conclusions.
Idiopathic nephrotic syndrome is the most common glomerular disease in children, with the vast majority of patients responding well to corticosteroids. The goal of the treatment is to achieve early remission, decrease the rate of relapses and reduce the cumulative exposure to steroids. Thus, the optimal duration and dosage of corticosteroids remain unsolved, with only a few randomized trials performed on this topic [5, 6, 14, 15]. Table 3 shows a summary of treatment regimens recommended by different guidelines [2, 12]. Although originally the ISKDC recommended prednisone dosage adjusted for BSA (60 mg/m2/24 h) [9], several studies of children with INS recommended an initial starting dosage of 2 mg/kg body weight per day [16-18]. Also, many authors and guidelines recommended either a weight-based or a BSA-based protocol, assuming them to be equivalent [2, 12].
Table 3
Summary of selected recommendations of treatment of first bout and relapse of idiopathic nephrotic syndrome in children
| Recommendations | First bout | Relapse |
|---|---|---|
| International Study of Kidney Disease in Children 1978 [9] | Prednisone 60 mg/m2/24 h for 4 weeks, followed by 40 mg/m2/48 h for 4 weeks | Prednisone 60 mg/m2/24 h until negative urine, followed by a single dose of 40 mg/m2/48 h |
| KDIGO Clinical Practice Guideline for Glomerulonephritis 2012 [2] | Prednisone 60 mg/m2/24 h or 2 mg/kg/24 h (max. 60 mg) in a single dose for 4-6 weeks, followed by a single dose of 40 mg/m2/48 h or 1.5 mg/kg/24 h (max. 40 mg) for 4-6 weeks. Thereafter prednisone dose is gradually tapered. Total duration of therapy – 12-24 weeks | Prednisone 60 mg/m2/24 h or 2 mg/kg/24 h (max. 60 mg) in a single dose until negative urine for 3 days, followed by a single dose of 40 mg/m2/ 48 h or 1.5 mg/kg/48 h (max. 40 mg) for at least 4 weeks |
| Canadian Society of Nephrology Commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis: management of nephrotic syndrome in children 2014 [26] | Prednisone 60 mg/m2/24 h or 2 mg/kg/24 h (max. 60 mg) in a single dose for 4-6 weeks, followed by a single dose of 40 mg/m2/48 h or 1.5 mg/kg/24 h (max. 40 mg) for 4-6 weeks. Thereafter prednisone dose is gradually tapered. Total duration of therapy – 12-24 weeks. Most patients should be treated for 12 weeks | Prednisone 60 mg/m2/24 h or 2 mg/kg/24 h (max. 60 mg) in a single dose until negative urine for 3 days, followed by a single dose of 40 mg/m2/ 48 h or 1.5 mg/kg/48 h (max. 40 mg) for at least 4 weeks |
| Recommendations of Polish Society for Paediatric Nephrology 2015 [12] | Prednisone 60 mg/m2/24 h or 2 mg/kg/24 h (max. 60 mg) in a single dose for 4-6 weeks, followed by a single dose of 40 mg/m2/48 h or 1.5 mg/kg/24 h (max. 40 mg) for 4-6 weeks. Thereafter prednisone dose is gradually tapered. Total duration of therapy – 24 weeks | Prednisone 60 mg/m2/24 h or 2 mg/kg/24 h (max. 60 mg) in a single dose until negative urine for 3 days, followed by a single dose of 40 mg/m2/48 h or 1.5 mg/kg/48 h (max. 40 mg) for 4 weeks. Thereafter prednisone dose is gradually tapered during 4 weeks |
| Recommendations of the Italian Society for Pediatric Nephrology 2017 [7] | Prednisone 60 mg/m2/24 h (max. 60 mg) in a single dose or divided into 2 doses for 6 weeks, followed by a single dose of 40 mg/m2/48 h (max. 40 mg) for 6 weeks. Total duration of therapy – 12 weeks | Prednisone 60 mg/m2/24 h (max. 60 mg) in a single dose or divided into 2 doses until negative urine for 5 days, followed by a single dose of 40 mg/m2/48 h (max. 40 mg) for 4 weeks |
| Current British practice (according to PREDNOS protocol) 2019 [6] | Prednisolone 60 mg/m2/24 h (max. 60 mg) for 4 weeks, followed by 40 mg/m2/48 h (max. 40 mg) for 4 weeks | Prednisone 60 mg/m2/24 h (max. 60 mg) until negative urine for 3 days, followed by 40 mg/m2/ 48 h (max. 40 mg) for 1 week, then taper by 10 mg/m2/day per week to complete a total of 4 weeks |
| Management of idiopathic childhood nephrotic syndrome in Sub-Saharan Africa: Ibadan consensus statement 2021 [8] | Prednisone 60 mg/m2/24 h (max. 60 mg) in a single dose for 6 weeks, followed by a single dose of 40 mg/m2/48 h (max. 40 mg) for 6 weeks. Thereafter prednisone dose is tapered at the rate of 10 mg/m2/week to 5 mg on alternate days. The dose of prednisone should be discontinued once tapered down to 5 mg on alternate days. Total duration of therapy – 16 weeks | Prednisone 60 mg/m2/24 h (max. 60 mg) in a single dose or divided into 2 doses until negative urine for 3 days, followed by a single dose of 40 mg/m2/48 h (max. 40 mg) for 1 week, then taper by 10 mg/m2/day per week to complete a total of 4 weeks |
The response to GS in patients with immune-mediated diseases is dose dependent, though optimal dosage to induce remission in INS is unknown. Also, the exact mechanism of action of GS in INS is not clear yet and may be multifactorial, involving both immunological and non-immunological actions [19, 20]. Experimental data suggest that glomerular podocytes may be a direct target of GS, as human podocytes express glucocorticoid receptors. The beneficial effect of GS might be due to direct protection of podocytes from injury and promotion of podocyte repair via upregulation of expression of the nephrin gene and stabilization of actin filaments and prevention of apoptosis [21, 22]. Doses necessary to stabilize podocyte cytoskeleton may be even lower than 2 mg/kg/24 h, as suggested by Mehls and Hoyer [23]. On the other hand, there are data suggesting that an increased cumulative dose during treatment of the first bout induces longer and more stable remission – probably influencing lymphocyte activity [19, 20].
Feber in 2009 found that the dosage of prednisone at 2 mg/kg per day versus 60 mg/m2 per day is significantly smaller for patients with weights < 30 kg. In the study by Feber the median dose per kilogram reached only 85% of the dose calculated per square meter and 91% of patients had been given a lower dose (ratio < 1.0) if the prednisone dose had been calculated as 2 mg/kg/24 h [10]. To reveal potential clinical significance of these calculations, Saadeh et al. analyzed retrospectively 56 children with an initial bout of INS treated with a weight-based regimen. The authors found that the underdosing percentage (defined as the difference between the BSA-based and the body weight-based dose) was significantly higher in those who became later frequent relapsers (16.6 ±7.9%) than in infrequent relapsers (8.7 ±9.8%) (p = 0.03). The underdosing percentage did not influence the initial response rate. The authors concluded that a higher initial dose could probably decrease the risk of further relapses, the need for repeated corticosteroid courses, and the need for the addition of other immunosuppressive drugs [11].
There are two prospective randomized Indian trials comparing outcomes in patients treated with these two regimens. Raman et al. compared 50 children treated with a weight-based and 50 children treated with a BSA-based protocol. The groups did not differ in terms of time to remission and relapse rate, but patients treated with the BSA-based regimen had a higher cumulative GS dose and higher incidence of hypertension [15]. Basu et al. analyzed 60 children in a randomized controlled study (first episode or relapse in infrequent relapsers). The authors also found that the weight-based regimen led to a significantly lower total cumulative steroid dose and lower number of adverse events without a difference in the 6-month relapse-free survival rate [24].
In contrast, our study produced opposite results in terms of the total GS dose. In our center, we used a prednisone dosing regimen in the 2nd month of 2 mg/kg/48 h, while in most recommendations and in the work of Basu and Raman, the prednisone/prednisolone dose in the 2nd month was 1.5 mg/kg/24 h [2, 9, 15, 24]. We think that this difference may have significantly affected the results. Hence, the conclusions of our work are different from the works of the mentioned authors. In addition, the Indian authors excluded patients with BSA > 1 m2 and body weight > 30 kg. By contrast, in our cohort there were 3 (7.5%) patients with BSA > 1 m2 and/or body weight > 30 kg. Of note, Italian authors proposed a simple equation (prednisone dose – 2 mg/kg + 8 mg) to estimate BSA-directed dosage from the patient’s body weight [25].
Corticosteroids have well-known metabolic side effects. High-dose and long-term therapy leads to growth retardation, central obesity, osteoporosis, cataract, and glaucoma. Side effects of systemic corticosteroids are dose- and time-dependent [20, 26]. In our cohort we did not observe any serious side effects except for arterial hypertension and increase in body weight. In both groups we observed a significant association between total amount of GS administered and increase in weight, BMI, as well as a negative impact on growth rate. A similar dose-dependent impact of GS on anthropometric parameters was found by other authors [27, 28]. The groups did not differ in mean numbers of relapses, but group II was characterized by a significantly lower dose of prednisone, a lower number of relapsing patients and a non-significantly longer period off steroids. Thus, we are deeply convinced that the therapy regimen currently used by us is favorable compared to the old one.
The study has some limitations including the small number of patients in both groups, non-randomized study design and mixed (retrospective and prospective) protocol, and the significant difference in age between the groups, which could have influenced the results. Of note, the higher dose in the second month (2 mg/kg/48 h) compared to most weight-based regimens and studies (i.e., 1.5 mg/kg/48 h) may have had the most significant effect on the final difference in dose and outcome in the two groups. We also did not perform bone density analysis in the studied group. Further studies are necessary to reveal the best possible therapy protocol for the first bout of idiopathic nephrotic syndrome.
Conclusions
The body surface area-based regimen of prednisone dosing in children with idiopathic nephrotic syndrome reduces exposure to steroids and risk of relapse, as well as increases days off steroids in the first year of the disease compared to a weight-based regimen with a high second-month dose.