Since the 1950s, vitamin K antagonists (VKA) have been used as oral anticoagulants for the treatment of venous thromboembolism and stroke prevention in patients with atrial fibrillation [1]. VKAs (e.g. warfarin, phenprocoumon, acenocoumarol) inhibit vitamin K epoxide reductase, which leads to attenuation of gamma-carboxylation of vitamin K-dependent coagulation factors (factor II, VII, IX, and X) and also anticoagulants protein C and protein S [2]. Due to the slow onset of action, narrow therapeutic window, increased bleeding risk, several food and drug-drug interactions, and need for frequent laboratory monitoring, there was a need for alternative agents.
During the last decade, direct oral anticoagulants (DOACs) have revolutionized the field of anticoagulation. Due to their fixed dosing, rapid onset of anticoagulant effect, predictable pharmacokinetics and dynamics, and non-inferiority to VKAs, DOACs are the recommended treatment for venous thromboembolism (VTE) and for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF) [3–5]. Dabigatran was the first DOAC that was approved in 2008 by the European Union and in 2011 by the Food and Drug Administration (FDA) [6]. Betrixaban was the last DOAC to be approved by the FDA, in 2017, and it is the only DOAC approved for extended duration prophylaxis for VTE in acute medically ill patients [7, 8]. Apixaban, rivaroxaban, and edoxaban are the other 3 approved DOACs [9–11].
This article is the first part of the narrative review on DOAC use in intensive care unit (ICU) patients, covering ‘Applied pharmacology’; the second article covers ‘Clinical evidence’.
SEARCH STRATEGY AND SELECTION CRITERIA
This is a non-structured narrative review conducted to provide an overview regarding applied pharmacology and the use of DOACs in ICU patients. The review was conducted by searching the PubMed, Embase, Medline, Google Scholar, and Clinicaltrials.gov databases. The search was conducted from Jan 1994 to Jun 2020 using the terms “dabigatran”, “rivaroxaban”, “apixaban”, “edoxaban” or “betrixaban”, “oral anticoagulants use in critically ill patients”, “DOACs/NOACs use in intensive care unit”, “oral anticoagulants in sepsis, shock and acute kidney injury”, and “oral anticoagulant management in perioperative periods”, to identify relevant papers, large randomized clinical trials, meta-analyses, and treatment guideline recommendations.
PHARMACOKINETICS AND PHARMACODYNAMICS
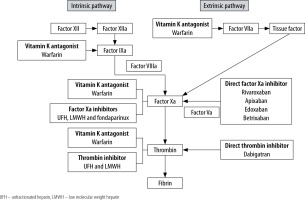
DAOCs, as the name indicates, act directly by inhibiting either thrombin (factor II) or activated factor X (factor Xa) of the coagulation cascade (Figure 1). Dabigatran is the only direct thrombin (factor II) inhibitor [6]. It binds to both free and fibrin-bound thrombin and prevents the conversion of fibrinogen to fibrin, thereby preventing thrombus formation [12]. It is produced by hydrolysis of prodrug (dabigatran etexilate) in the plasma and liver [13]. Dabigatran can be taken with or without meals and is rapidly absorbed. Even though food does not have any effect on the bioavailability of dabigatran, it does delay its maximum plasma concentration by 2 hours [14]. Oral bioavailability of dabigatran is 6-7%, which is independent of prodrug dose [15]. It has a half-life of 12-14 hours, and around 80% of it is eliminated by the kidneys [16] (Table 1).
TABLE 1
Pharmacological characteristics of direct acting oral anticoagulants
Rivaroxaban is a selective inhibitor of factor Xa, has high specificity for the factor Xa active site, and acts independently of antithrombin [15]. It is rapidly absorbed after oral administration and has a reported bioavailability of up to 100%, which varies with dosage and administration with food intake [17]. Co-administration with food increases the bioavailability and increases the maximum plasma concentration [18]. Rivaroxaban reaches its maximum plasma concentration in 2-4 hours and has a half-life of 5-9 hours. It is eliminated by both renal and hepatobiliary systems [17] (Table 1).
Apixaban inhibits both free and bound factor Xa and prevents thrombin formation from prothrombin [19]. It is also rapidly absorbed after oral administration, and its bioavailability is not affected by co-administration with food [20]. Its level peaks in 1-2 hours and has a half-life of 8-15 hours [21]. It is mainly metabolized by the hepatic system, and around one-third of it is cleared by the renal system [22].
Edoxaban is a highly selective factor Xa-inhibitor, approximately 10,000 times more selective for factor Xa as compared to thrombin [23]. It is rapidly absorbed after oral administration, has a bioavailability of 58–62%, reaches peak plasma concentration within 1–2 hours, has a half-life of 10–14 hours, and is eliminated both by the kidneys and hepatic metabolism [24, 25]. Co-administration with food or hepatic impairment does not affect its systemic exposure, but dose adjustment is needed in cases of renal dysfunction [24–26].
Betrixaban is a very potent factor Xa-inhibitor and inhibits both free and bound factor Xa [27]. It has a bioavailability of 34% and reaches its peak plasma concentration within 3–4 hours. It has a long half-life (37 hours), low renal clearance, and no metabolism by cytochrome P450 system [28, 29]. It is eliminated unchanged largely by the biliary system (82–89%), and a very small amount is excreted in the urine [28, 29].
EFFECT OF DIRECT ORAL ANTICOAGULANTS ON COAGULATION PARAMETERS
One of the major advantages of DOACs is the absence of the necessity to perform routine laboratory monitoring. However, in complex or critical situations, limited availability of specific coagulation tests can be challenging. Routinely used coagulation tests, activated partial thrombin time (aPTT) and prothrombin time (PT), are prolonged by most DOACs but such tests are of limited utility due to unpredictable relation to plasma concentration of the DOACs [30, 31].
At the therapeutic level, dabigatran causes a prolongation of aPTT, but a normal aPTT does not completely exclude its anticoagulant effect [31, 32]. The effect of dabigatran can be measured accurately by diluted thrombin time (dTT) and ecarin clotting time (eCT) [33, 34]. Both tests have shown a strong linear relationship to dabigatran concentration, making them useful in critical situations [33]. Unfortunately, these tests are not readily available in every hospital. Rivaroxaban prolongs PT, but the degree of prolongation does not have a linear relationship to dose and is dependent on the reagent used [35]. Normal PT is suggestive of zero or minimal activity of rivaroxaban [33]. Other factor Xa-inhibitors can prolong PT but in an unpredictable pattern, which is not helpful in assessing the anticoagulant activity of those DOACs [36, 37].
Anti-Xa assay, primarily designed to measure the effect of unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH), is available in most hospitals and provides qualitative testing for factor Xa-inhibitors. Accurate and quantitative testing of factor Xa-inhibitors can be done by calibrated chromogenic anti-Xa assays; unfortunately, its availability is still very limited [33, 38].
Rotational thromboelastometry (ROTEM) is a point of care system available in emergency and operating rooms to monitor coagulation and to guide transfusion of platelets, plasma, and fibrinogen for severe bleeding (post traumatic or obstetrical) and during surgeries (hepatic or cardiac). Studies have shown a linear relationship between clotting time measured by ROTEM and increasing plasma concentration of DOACs including factor Xa-inhibitors and dabigatran. This offers reliable clinical potential of ROTEM to predict the DOAC concentration in cases of severe bleeding [39, 40].
INDICATIONS AND DOSING OF DIRECT ORAL ANTICOAGULANTS
All approved indications and standard dosing for each clinical condition of all DOACs are shown in Table 2.
TABLE 2
Indications and dosing of direct acting oral anticoagulants
[i] NVAF – nonvalvular atrial fibrillation, CrCl – creatinine clearance, DVT – deep venous thrombosis, PE – pulmonary embolism, BID – bis in die, OD – once daily, ESRD – end stage renal disease, CV – cardiovascular, MI – myocardial infarction, CAD – coronary artery disease, PAD – peripheral artery disease, P-gp – permeability glycoprotein
Dabigatran, rivaroxaban, apixaban, and edoxaban are approved for prevention of stroke and systemic embolization in patients with NVAF, as well as for the treatment of deep venous thrombosis (DVT) and pulmonary embolism (PE). Dabigatran, rivaroxaban, and apixaban are also indicated for DVT or PE prophylaxis following hip or knee replacement surgery, while betrixaban is the only DOAC that is approved for prophylaxis for prevention of VTE in acutely ill medical patients but not in ICU patients.
DIRECT ORAL ANTICOAGULANT DOSING IN SPECIFIC MEDICAL CONDITIONS
There is always the possibility of various ongoing pathophysiological derangements in critically ill patients, including impaired renal and/or hepatic function. Table 3 highlights important precautions and concerns while using DOAC in ICU patients.
TABLE 3
Important precautions and concerns while using direct oral anticoagulant therapy in critically ill patients
Renal function impairment
All DOACs are eliminated by the renal system to some extent [16]. Based on pharmacokinetic studies and clinical studies with relatively small sample sizes FDA has approved the use of apixaban, rivaroxaban, dabigatran, and edoxaban in patients with creatinine clearance (CrCl) as low as 15 mL min−1. Studies have shown a gradual increase in the levels of DOACs as renal function deteriorates [41–43]. This leads to increased risk of bleeding in patients with advanced kidney disease and patients on haemodialysis who are already at high risk of bleeding due to haemodialysis-related factors (i.e. heparin use during dialysis, vascular access cannulation, etc.) [16, 44].
The dosing and CrCl used for exclusion from phase III DOACs trials were based on the Cockcroft-Gault equation, even though the Cockcroft-Gault formula can overestimate true CrCl by 10% to 40%. Hence, this formula should be used for DOAC dosing [16]. Among all the DOACs, dabigatran is the one that is highly dependent on kidneys for its clearance (80%), and it is the only DOAC that can be removed by dialysis [16, 45]. The FDA-approved dose for Dabigatran in patients with CrCl > 30 mL min−1 is 150 mg twice daily, but guidelines advise caution with dabigatran use in patients with CrCl between 30 and 50 mL min−1 due to increased risk of bleeding [46, 47]. For patients with CrCl between 15 and 30 mL min−1, 75 mg twice daily is the FDA approved dose. Dabigatran can be removed effectively (52–77%) by intermittent haemodialysis but with a rebound effect of up to 87% within 2 hours of intermittent haemodialysis completion [42, 45, 48]. Continuous renal replacement therapy can help to attenuate the rebound effect [45].
Dose adjustment is also recommended for rivaroxaban because the kidneys eliminate 36% of the parent drug [49]. Rivaroxaban is approved by the FDA at a dose of 20 mg once daily in patients with CrCl > 50 mL min−1 and 15 mg once daily for those with CrCl of 15–50 mL min−1, for stroke prevention, but it is not recommended for use in patients with CrCl < 15 mL min−1 or those on haemodialysis [11, 16, 50].
No dose adjustment is recommended for apixaban for any degree of renal impairment [16]. Based on a small single-centre study, FDA labelling recommends no change in apixaban dosing for patients on haemodialysis [43]. Only 6.7% of apixaban is cleared by a 4-hour session of haemodialysis [43].
Edoxaban is one of the newest approved factor Xa inhibitors. Approximately 50% of it is cleared by kidneys, and mild to severe renal impairment can lead to 33% to 72% increased drug exposure [24]. No dosage adjustment is necessary in patients with CrCl ≥ 51 mL min−1. It is recommended that the dose is decreased to 30 mg once daily from 60 mg once daily if the CrCl is 15–50 mL min−1. Edoxaban is not recommended for use in patients with CrCL < 15 mL min−1 [24, 25].
As mentioned above, betrixaban has very low renal clearance, which suggests the possibility of safe use in patients with severe renal impairment, CrCl ≥ 15 to < 30 mL min−1 [29]. In the pivotal APEX trial (The Safety and Efficacy of Full Versus Reduced Dose Betrixaban in the Acute Medically Ill VTE Prevention with Extended Duration Betrixaban), patients with severe renal insufficiency had higher plasma concentration of betrixaban after 80 mg and 40 mg as compared to patients with normal renal functions. This increase in plasma concentration was not associated with an increase in the major bleeding [51].
DOACs use is not recommended in cases of acute kidney injury due to a lack of studies in these patients [52].
Hepatic function impairment
DOACs undergo a wide range of biotransformation within the hepatic system. Apixaban is the most reliant on hepatic metabolism, followed by rivaroxaban, edoxaban, dabigatran, and betrixaban [53, 54]. Guidelines recommend avoiding the use of DOACs in patients with Child-Turcotte-Pugh class C cirrhosis [52]. However, there have been no consensus statements for patients with relatively mild liver disease or abnormalities of liver function tests.
Extremes of body mass
Obesity is a risk factor for both atrial fibrillation and venous thrombosis [55, 56]. As noted above, DOACs are now considered the first choice for prevention of thromboembolic complications of NVAF and treatment for VTE. Unfortunately, data are limited for the efficacy and safety of DOAC use in patients at extremes of weight. Current FDA labelling does not recommend dose adjustment of these agents for either an abnormally low or high body mass index (BMI) [57]. The International Society on Thrombosis and Haemostasis (ISTH) recommended standard DOAC doses for patients with BMI ≤ 40 kg m−2 and mass ≤ 120 kg [58]. The ISTH does not recommend use of DOACs in morbidly obese patients with a BMI > 40 kg m−2 or mass > 120 kg due to limited clinical data [58]. As per ISTH recommendations, if a DOAC is used in morbidly obese patients, peak and trough laboratory monitoring (anti-factor Xa level for factor Xa-inhibitors; eCT or dTT for dabigatran) should be checked. If measured laboratory tests fall below the expected range, switching to VKA was recommended rather than adjusting the DOAC dosage [58].
Since ISTH recommendations in 2016, new data has evolved regarding the use of DOACs, especially apixaban and rivaroxaban, in patients with extremes of weight. Studies have shown significantly lower bleeding and similar or better efficacy of DOACs as compared to warfarin in patients with morbid obesity (BMI > 40 kg m-2) [59–61]. Recently published meta-analyses by Malik et al. [62] and Kido et al. [63] gave assuring results and did not reveal problems in the use of DOACs in patients at extremes of weight. Unfortunately, most of the recent studies are retrospective in nature and only evaluated apixaban and rivaroxaban in comparison to warfarin [62, 63].
ADVERSE EFFECTS
A comparison between outcomes of randomized clinical trials and real-world studies of routine clinical practice has provided better insight into the safety of DOACs [64, 65]. Bleeding is the major side effect of any blood-thinning medication, including DOACs. As compared to VKA, reported rates of major bleeding due to DOACs are either similar or reduced [64–66]. DOACs also have reduced intracranial bleeding rates but increased gastrointestinal bleeding, as compared to VKA [64, 66]. Among the DOACs, relative to VKA, apixaban has the lowest hazard ratio for major bleeding followed by dabigatran [64]. Compared to VKA, DOACs have not shown significantly different rates of mortality and recurrent major bleeding at 30 days [11, 57, 64, 66]. Other reported adverse effects of DOACs include dyspepsia, transient hepatic impairment (dose-dependent in case of dabigatran), constipation, diarrhoea, headache and lightheadedness, gross haematuria (especially with edoxaban), and peripheral oedema (with apixaban) [6, 27, 67–70].
Drug–drug interactions
There are various drugs which can influence the drug level of DOAC, when used concurrently (Table 4). Dabigatran and dabigatran etexilate are not metabolized by the cytochrome P450 system (CYP) and they do not inhibit CYP [6, 12]. Co-administration of dabigatran etexilate with CYP substrates does not alter the pharmacokinetics and pharmacodynamics of either drug [6, 12, 71]. Because dabigatran is a substrate for P-glycoprotein (permeability glycoprotein, P-gp), there is a potential for drug-drug interaction with co-administration of P-gp inhibitors or inducers [71]. Co-administration of dabigatran with strong inhibitors of P-gp (cyclosporine, dronedarone, itraconazole, and systemic ketoconazole) is contraindicated or not recommended (tacrolimus) [13, 71, 72].
TABLE 4
Effect of various drugs on the drug level of direct oral anticoagulants
Close monitoring and caution should be exercised with co-administration of mild to moderate inhibitors of P-gp (amiodarone, posaconazole, quinidine, ticagrelor, and verapamil) [13, 71, 72]. Patients with severe renal dysfunction should not be on dabigatran and P-gp inhibitors together because P-gp inhibitors can increase dabigatran levels and lead to life-threatening bleeding [42]. Also, P-gp inducers (e.g. phenytoin, carbamazepine, rifampin) should be avoided in patients who are on dabigatran, to prevent a decrease in dabigatran level [13, 71, 72]. Co-administration of dabigatran with an H2-blocker does not affect its absorption, but administration with proton pump inhibitor can decrease dabigatran exposure by 30% [69, 73].
Drug-drug interactions of rivaroxaban and apixaban are mediated by CYP3A4 and P-gp transport systems because these systems are involved in metabolism and excretion of both [17, 74, 75]. Rivaroxaban and apixaban are not inducers or inhibitors of CYP enzymes and do not interfere with the transport of P-gp substrates, as demonstrated by no clinically relevant interaction when co-administered with substrates (e.g. digoxin, atorvastatin) of these systems [74–77]. Co-administration of rivaroxaban and apixaban with strong inhibitors (e.g. ritonavir, ketoconazole, itraconazole) of both CYP3A4 and P-gp should be avoided because it can increase their plasma concentration [77–79]. Strong inhibitors (e.g. clarithromycin) of either CYP3A4 or P-gp, or moderate inhibitors (e.g. erythromycin, fluconazole) of both of these pathways, moderately affect rivaroxaban and apixaban exposure, and such interaction is not considered clinically relevant for dose adjustment [17, 22, 70, 79]. Concomitant administration of rivaroxaban and apixaban with strong CYP3A4 and P-gp inducers (e.g. rifampicin) can lead to decreased exposure of these DOACs and is generally not recommended [17, 71, 80].
Changes in gastric pH by H2-receptor antagonists (ranitidine, famotidine), antacids, and proton pump inhibitors (e.g. omeprazole) do not affect the pharmacokinetics of rivaroxaban and apixaban [81–83]. Co-administration of rivaroxaban with aspirin, clopidogrel, enoxaparin, and warfarin does not alter the pharmacokinetics of rivaroxaban [81, 84, 85].
Edoxaban is cleared mainly by the P-gp transporter system, which mediates its drug-drug interactions [86]. Edoxaban is minimally metabolized by the CYP3A4 system, and its inhibitors or inducers do not affect dabigatran levels [24, 86, 87]. Co-administration of edoxaban with strong P-gp inhibitors (verapamil, ritonavir, dronedarone) results in > 50% increased exposure of edoxaban. It is recommended that the dose of edoxaban is decreased or agents are considered with zero to minimal effect on the P-gp system [24, 86, 88]. Use of amiodarone, a P-gp inhibitor, results in a modest increase in edoxaban exposure and should be used with caution, or alternative agents should be considered in case of additional interactions [24, 86, 88]. Co-administration with P-gp inducers (e.g. rifampin, phenytoin) should be avoided due to decreased exposure and consequently decreased efficacy [24, 86, 87]. Co-administration with proton pump inhibitor (e.g. esomeprazole) does not result in a clinically significant effect on the total exposure [24].
Less than 1% of betrixaban is metabolized by the CYP enzyme system, and it does not inhibit or induce CYP enzymes, lowering the rates of drug-drug interactions [28]. Like dabigatran and edoxaban, betrixaban is a substrate of the P-gp transport system, and its drug interactions are mediated by it. A dose reduction to 40 mg is considered appropriate when it is co-administered with strong P-gp inhibitors [28, 89].
REVERSAL AGENTS
Specific reversal agents
Direct thrombin inhibitor reversal agent
Idarucizumab is a monoclonal antibody approved by the FDA to reverse the anticoagulant effect of dabigatran. It binds both free and thrombin-bound dabigatran and has > 350 times the binding affinity for dabigatran than thrombin [90, 91]. It has been shown to completely reverse the dabigatran anticoagulant effect in non-bleeding healthy volunteers, patients with major bleeding, or patients who need urgent surgical intervention.
The recommended dose of idarucizumab is 5 g. Two vials of 2.5 g can be administered either as two consecutive infusions or by infusing both vials consecutively via syringe. It can reverse 100% of the dabigatran effect as assessed by dTT or eCT [90, 91] (Table 5).
TABLE 5
Reversal agents and their dose for direct acting oral anticoagulants
Factor Xa-inhibitor reversal agent
In May 2018 the FDA approved andexanet alfa as a reversal agent for factor Xa inhibitors, rivaroxaban, and apixaban [92]. Andexanet alfa is a recombinant factor Xa protein that binds to factor Xa inhibitors and allows intrinsic factor Xa to function in the clotting cascade. It has been studied only in patients receiving apixaban or rivaroxaban [93, 94]. It has a short half-life, so it is administered as a bolus followed by 2-hour infusion. It can reduce anti-factor Xa activity by 92–94% and regeneration of thrombin within 2–5 minutes [93, 94] (Table 5).
Non-specific agents
In the absence of a specific reversal agent, non-specific agents such as activated or unactivated prothrombin complex concentrates (PCC) can be considered to control bleeding or in cases of urgent surgical interventions [95]. In patients who are on dabigatran, an activated PCC (aPCC) such as factor 8 inhibitor bypassing activity (FEIBA) can be considered for use. 4-factor or 3-factor PCC are reasonable options if an aPCC is unavailable [95–97]. In patients on factor Xa inhibitors, 4-factor or 3-factor PCC can be used [95–97]. 4-factor PCC (4-PCC) is most widely used because it is most effective among other blood products for DOAC-related bleeding [98] (Table 5). 4-PCC use was not shown to reverse the dabigatran effect on aPTT, eCT, or thrombin time, but it did show normalization of prothrombin time [96]. Normalization of coagulation parameters by use of fresh frozen plasma or PCC has been reported in cases of rivaroxaban overdose, but it does not imply the reversal of the anticoagulant effect of the DOAC [99, 100].
CONCLUSIONS
Currently, dabigatran is the only approved drug that has direct thrombin (factor II) inhibition; while rivaroxaban, apixaban, edoxaban, and betrixaban are selective inhibitors of factor Xa. All except betrixaban are approved for the treatment of venous thromboembolism as well as for the prevention of stroke in non-valvular atrial fibrillation, while betrixaban is approved only for the prevention of venous thromboembolism in acutely ill medical patients but not in ICU patients. In clinical use, DOACs do not require regular monitoring by routinely used coagulation tests, including activated partial thrombin time and prothrombin time, due to the unpredictable relation of these test results to the plasma concentration of the drug. For patients with renal dysfunction, dose modification must be considered for all DOACs as per creatinine clearance. In critically ill patients, the use of various concurrent medications might influence the plasma drug level of DOACs. Bleeding is a major concern for patients who are on anticoagulants, and evidence so far suggests that DOACs are associated with less major bleeding and reduced risk of intracranial bleeding but a higher risk of gastrointestinal bleeding. Due to lack of data, DOAC use should be avoided during the critical phase of illness given the unpredictable pharmacokinetic profile and the need for invasive interventions. Patients should be managed with parenteral anticoagulation during the critical phase, and DOACs can be resumed or initiated as soon as the patient is clinically stable and there is no need for invasive procedures.