Introduction
In spite of considerable research, preeclampsia still remains probably the most mysterious and interesting complication of pregnancy. It affects 2–3% of pregnant women, mostly nulliparous patients [1]. Although the common definition of preeclampsia, including the new onset of hypertension and proteinuria in the second half of pregnancy, is still widely accepted, different societies have modified certain criteria of the disease [1–3]. In 2013, the American College of Obstetricians and Gynecologists (ACOG) decided not to necessarily include proteinuria in the diagnosis of preeclampsia [2]. According to them, preeclampsia may be diagnosed if new onset of hypertension after 20 weeks of pregnancy is associated with at least one of the following: 1) thrombocytopenia 2) renal failure, 3) liver failure, 4) pulmonary edema or 5) neurological and/or visual disturbances [2]. Similarly, the Society of Obstetricians and Gynecologists of Canada (SOGC) decided that proteinuria is not an essential criterion of preeclampsia, which may be diagnosed also if the presence of hypertension in the second half of pregnancy is accompanied by a different complication or adverse condition [3]. They included, besides the complications mentioned above by ACOG, the consequences of feto-placental disturbances, such as intrauterine growth restriction (IUGR), oligohydramnios, and abnormal results of the fetal umbilical artery and/or ductus venosus Doppler [3].
These modifications indicate the complex and multiorgan nature of the disease as well as the crucial role of the placenta in the pathophysiology of preeclampsia. Such an approach should increase the diagnostic accuracy.
Currently, the most widely accepted theory for the development of preeclampsia is the “two- stage theory” [4]. According to this theory, the first stage of the disease is reduced placental perfusion and the second is generalized maternal endothelium dysfunction [4]. The main cause of reduced placental perfusion is poor placentation as a consequence of impaired trophoblast invasion into the lumen and the walls of spiral arteries. Endothelium damage is probably caused by antiangiogenic factors excessively produced by the poorly perfused placenta and transferred to the maternal circulation [4]. These factors, including soluble fms-like tyrosine kinase 1 (sFlt1) and soluble endoglin (sEng), contribute to endothelium damage and dysfunction by binding, respectively, vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)-β1, which are required to maintain normal endothelium function [5]. The consequence of maternal endothelium damage may be the different symptoms and complications characteristic of preeclampsia, such as hypertension, neurological symptoms, proteinuria and thrombocytopenia. The increased production of antiangiogenic factors in the placentas of patients who developed complications was observed many weeks before the onset of the disease [6].
This “two-stage theory” for the development of preeclampsia mainly explains the pathophysiology of the early-onset disease that develops before 34 weeks of gestation, whereas late-onset preeclampsia (> 34 weeks), including term preeclampsia (≥ 37 weeks), may have a different etiology [7]. The late-onset disease may be attributed to the primary cardiovascular system pathology, including angiopathy and/or decreased cardiac output without the primary role of poor placentation in the onset of the disease [7, 8]. The possible risk factors of this form of preeclampsia include pregestational diabetes, particularly class F or R, hyperlipidemia, obesity and chronic hypertension [7, 9].
The purpose of this review was to summarize and emphasize the role of the serum assessment of many different popular biochemical markers, including new ones, in the prediction, diagnostics and clinical management of preeclampsia. The recent enormous scientific development in this field seems to be critical for the change in clinical management of preeclampsia in the future. This is why it seems important to bring this topic closer to the attention of clinicians.
Material and methods
Search process
The Medline (PubMed) database was searched from January 2004 to April 2018 to identify articles on use of biochemical tests in preeclampsia. There was no restriction on language. The following key words and subject headings were used in the search: “preeclampsia”, “biochemical tests”, “antiangiogenic factors” , sFlt-1, soluble endoglin (sEng), PlGF, pregnancy-associated plasma protein A (PAPP-A) and placental protein 13 (PP 13).
Inclusion criteria
The following inclusion criteria were applied:
– full text articles assessing the value of biochemical tests in prediction of preeclampsia in first, second and third trimesters of pregnancy,
– full text articles assessing the value of biochemical tests in diagnosis of preeclampsia,
– full text articles assessing the value of biochemical tests in management of preeclampsia,
– full text articles assessing the value of biochemical tests in monitoring of preeclampsia treatment by new methods.
Studies were included if they reported original data only.
Exclusion criteria
Articles were excluded from the final review if:
– they concerned not only preeclampsia but other types of hypertension in pregnancy or other pregnancy pathologies,
– they, as full text articles, were written in a language other than English or Polish,
– they did not analyze the prediction value of biochemical tests in a specific period of time at the first trimester (11–13 weeks gestation).
Results
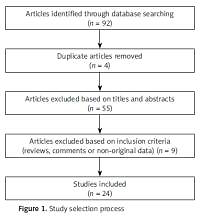
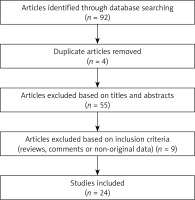
Of the 92 studies identified, 24 were included in our review. Details on the study selection process are shown in the Figure 1.
Quality of included studies
All included studies were prospective. All studies had adequately described population selection, tests, measurement used, and outcomes. In the studies concerning prediction of preeclampsia in first trimester all recruited women were between 11 and 13 weeks gestation.
Use of biochemical markers in preeclampsia prediction in the first trimester of pregnancy
Nowadays, as in the case of fetuses with trisomies, we can almost accurately predict the occurrence of preeclampsia in early pregnancy [6, 10]. The most popular first trimester biochemical markers of preeclampsia are as follows: 1) pregnancy-associated plasma protein A, 2) placental growth factor (PlGF), 3) soluble fms-like tyrosine kinase 1 (sFlt1), 4) placental protein 13 (PP13), and 5) triglycerides [10, 11].
Pregnancy-associated plasma protein A (PAPP-A) is an enzyme, a metalloproteinase, produced by syncytiotrophoblasts, responsible for the cleavage of complex insulin-like growth factor (IGF)-binding protein (IGFBP). The consequence of its activity is release of IGF from the complex [12, 13]. IGF stimulates placental growth and development [14]. In the first trimester of pregnancies complicated later by preeclampsia, the serum concentration of PAPP-A has been found to be significantly lower than that in pregnancies in which preeclampsia did not develop [10, 11]. In clinical practice the serum level of PAPP-A should be presented in percentiles or in multiples of median (MoM) [11, 14]. Further, the decreased concentration of PAPP-A at 11–13 weeks of pregnancy has a 44% and 37% detection rate of early and late preeclampsia, respectively, with a false positive rate (FPR) of 5% [15, 16].
Placental growth factor (PlGF) is a protein, a vascular endothelial growth factor (VEGF) homolog, produced by cytotrophoblasts. Similar to VEGF, it has proangiogenic activity, and thus promotes placental growth and development [14, 17]. The maximum serum concentration of PlGF is observed at 29–32 weeks of pregnancy [17]. In pregnancies complicated by preeclampsia the PlGF level in the first trimester of pregnancy decreased [18–20]. In clinical practice, the serum concentration of PlGF is expressed in percentiles or in MoM. The use of PlGF alone in the prediction of preeclampsia in the first trimester of pregnancy has a detection rate of 59% and 41% for early and late preeclampsia, respectively, with a false positive rate of 5% [15, 16].
Soluble fms-like tyrosine kinase 1 (sFlt1) is a circulating protein and a soluble form of the vascular endothelial growth factor 1 receptor (VEGFR1), produced by syncytiotrophoblasts [5]. It is an antiangiogenic factor that inhibits proangiogenic activity of VEGF, including its protective effect on the endothelium. It may bind both PlGF and VEGF, which has a negative influence on the placenta and the maternal endothelium [5]. In patients who later developed preeclampsia the serum concentration of sFlt-1 in the first trimester was significantly higher than in pregnant women, who remained healthy throughout their pregnancy [21].
Placental protein 13 (PP13) is a protein produced by trophoblasts. It is an immunomodulator that induces the apoptosis of T cells and macrophages, and plays an important role in the early placental and maternal-placental interface development [22–24]. As for PAPP-A and PlGF, in patients who developed preeclampsia, the serum level of PP13 was significantly lower than that in patients who remained normotensive throughout their pregnancy [11, 25]. In a meta-analysis, a low level of PP13 in the first trimester of pregnancy was found to be more predictive for the occurrence of early preeclampsia than that of late preeclampsia [11].
The common feature of all of the above biochemical markers of preeclampsia (PAPP-A, PlGF, sFlt-1, and PP 13) is their placental origin. Other, non-placental, markers of preeclampsia development are triglycerides.
The use of biochemical tests in the first trimester of pregnancy together with the other methods, including ultrasound, has been found to be very effective in the prediction of preeclampsia, mainly early onset or preterm preeclampsia [10, 14]. Nowadays it is well accepted to use the measurement of PAPP-A and PlGF together with the assessment of the mean uterine artery pulsatility index (PI) and the mean arterial pressure (MAP) at 11–13 weeks of pregnancy in the screening of preeclampsia [10, 14]. Using this method, we can predict early-onset preeclampsia (< 34 weeks) and preterm preeclampsia (< 37 weeks) in 93% and 61% of cases, respectively, with a false positive rate of 5% [15, 16]. At the same time, the prediction of term preeclampsia (> 37 weeks) is considerably poor (only 38%) [15, 16].
Prediction of preeclampsia in the second and third trimester of pregnancy using biochemical markers
In the second half of pregnancy, we can predict the development of preeclampsia by assessing the serum level of angiogenic and antiangiogenic markers including PlGF, sFlt-1, and soluble endoglin (sEng). The increased levels of serum sFlt-1 and sEng were found to be significantly higher in the second and third trimester of pregnancy in patients who developed preeclampsia than in pregnant women who remained healthy throughout their pregnancy [26, 27]. This correlation mainly concerned preterm preeclampsia [26, 27]. Conversely, those women who developed preeclampsia had significantly lowers levels of PlGF in the second and third trimesters than patients who remained normotensive throughout their pregnancy [26, 27]. The levels of PlGF in women who developed preterm preeclampsia were lower than in patients with the term onset of the disease [27]. In one of the studies, the highest predictive value, particularly for the development of preterm preeclampsia, was found for sFlt-1/PlGF ratio [26]. The decreased or increased concentration of the angiogenic or antiangiogenic factors, respectively, is found usually already 5–6 weeks before the onset of the disease [26]. The same phenomenon is even more evident for the sFlt-1/PlGF ratio, which increases a few weeks before the presentation of the disease. Therefore, the sFlt-1/PlGF ratio may be regarded as an index of angiogenic imbalance [28]. PROGNOSIS study showed a very high negative predictive value (NPV) of 99.3% for the occurrence of preeclampsia within a week if the sFlt-1/PlGF ratio was ≤ 38 and a positive predictive value (PPV) of 36.7% for the onset of the disease within 4 weeks if the ratio was > 38 [29]. A summary of the use of different biochemical markers in preeclampsia is presented in the Table I.
Use of biochemical tests in preeclampsia diagnosis
At present, in spite of the considerable development in the research and clinical practice regarding preeclampsia, the diagnosis of this disease is still sometimes difficult. It is usually associated with a heterogenic clinical presentation and the sudden onset of the disease. In many cases not all maternal organs are involved in the pathophysiology of preeclampsia. Some patients present with more placenta-related symptoms, and others with more maternal ones [7]. Thus, according to some gynecological associations, now it is not necessary to include proteinuria as a criterion of the disease [2, 3].
The significant progress in the efficacy of the diagnosis of preeclampsia, a particularly atypical form of the disease or the related complications, may be the use of the serum measurement of the angio- and antiangiogenic markers, including PlGF and sFlt-1. The most accurate one of these markers is the sFlt-1/PlGF ratio [6, 30, 31]. Before 34 weeks of gestation, an sFlt-1/PlGF ratio of ≥ 85 was found to have a sensitivity/specificity of 88%/99.5% in the diagnosis of preeclampsia [30]. The above ratio also predicts the occurrence of maternal and fetal complications of preeclampsia within 2 weeks very well [32]. At ≥ 34 weeks of gestation, the highest sensitivity/specificity was found for the sFlt-1/ PlGF ratio of ≥ 110 (58.2 and 95.5%, respectively) [30]. The measurement of the sFlt-1/PlGF ratio also plays an important role in ruling out preeclampsia [6, 30]. Both before and after 34 weeks, if the ratio is ≤ 33, the sensitivity/specificity for ruling out of preeclampsia is very high, 95%/94% (< 34 weeks) and 89.6%/73.1% (≥ 34 weeks) [30].
Additionally, the sFlt-1/PlGF ratio measurement may be helpful in the diagnosis of different subforms of preeclampsia, such as the HELLP syndrome, as well as the differential diagnosis of HELLP with other causes of thrombocytopenia during pregnancy [33].
Use of biochemical tests in preeclampsia management
Although preeclampsia is very often a dangerous condition for both a mother and a fetus, the severity of the disease varies. Besides the degree of hypertension and proteinuria if present, the degree of placental insufficiency, and the coexistence of different complications of preeclampsia, including hepatic or neurological ones, another possible marker of the intensity of the disease is the sFlt-1/PlGF ratio [34]. For example, in patients with preeclampsia in whom the abovementioned ratio was < 85 only 15.8% required delivery within 2 weeks in comparison to 86% of women with a ratio > 85 [32]. This finding indicated a positive correlation between the ratio and the severity of the disease. The results of another study, which showed that if the sFlt-1/PlGF ratio was very high, above 655, only 5.9% of the patients remained undelivered within a week, which confirmed the abovementioned findings [35]. These as well as other results showed the significant utility of this biochemical test, including also the need for hospitalization [32, 36]. In the first study on this topic, the sFlt-1/PlGF ratio was proven to be a helpful clinical marker for determining whether to hospitalize a patient with preeclampsia or not [36]. Another helpful role of an assessment of this ratio is in determining the occurrence of both maternal and fetal complications during the course of preeclampsia. A significantly high sFlt-1/PlGF ratio was found in women with preeclampsia who developed different complications of the disease [32].
Currently, the most important utility of the assessment of the sFlt-1/PlGF ratio in women with preeclampsia seems to be ruling out the disease [34]. Therefore, it has already been implemented in clinical practice in some European countries, such as Germany, England and Italy [34]. It seems to be particularly helpful in various types of hypertensive disorders of pregnancy, idiopathic cases of placental insufficiency, and different dysfunctions of the liver and different causes of thrombocytopenia. All these pathologies may be a clinical element of preeclampsia.
Use of sFlt-1/PlGF ratio in the monitoring of preeclampsia treatment by new methods
One possible new treatment of preeclampsia is apheresis [37, 38]. The aim of this treatment is to remove sFlt-1 from the maternal circulation. The primary efficacy of the treatment with a significant, transient reduction of the sFlt-1/PlGF ratio was found [38].
Conclusions
Nowadays, various biochemical tests play a crucial role in the prediction of preeclampsia. Both in the first trimester and in the later trimesters, they are very helpful in predicting preeclampsia, particularly in the cases of an early onset of the disease. In the first trimester, both PAPP-A and PlGF assessments, together with other prediction methods, form the basis of a prediction test for preeclampsia. In the second and third trimesters the most helpful in the prediction of the disease seems to be the sFlt-1/PlGF ratio. Recently, the measurement of this ratio has gained increasing popularity not only as a prediction test but also as a diagnostic test with some utility in the management of preeclampsia.