Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed cancers in men. In the United States and many Western European countries, the incidence of PCa outweighs the incidence of other malignancies among the male population [1]. PCa remains the second leading cause of death in males. Since 1985, a gradual increase in PCa-related mortality has been observed worldwide [2].
Laparoscopic radical prostatectomy (LRP) was first introduced by Schuessler et al. in 1997 [3]. It is a minimally invasive surgical technique that is offered to men with localized PCa. Endoscopic treatment of PCa offers good visibility of the surgical field, which leads to more accurate identification of anatomical structures [4–6].
Despite the technical improvement, it should be remembered that LRP is associated with the risk of intra- and postoperative complications and requires surgical skills in laparoscopic techniques. This method represents a challenge for many urologists, especially considering the learning curve, procedure difficulty, and time needed to perform vesicourethral anastomosis (VUA) [7]. The quality of VUA is associated with complications that could significantly affect the patient’s urinary-related quality of life, such as urethral stricture, bladder neck contracture (BNC), or urinary incontinence. The most popular and widely used VUA technique is the method introduced in 2003 by Van Velthoven et al. [8]. Despite its effectiveness, this method is time-consuming (average anastomosis time is 35 min) and not free of complications. Many VUA methods during LRP aim to simplify the surgical procedure and reduce the time of surgery [9–16]. In 2009, an alternative technique for laparoscopic running VUA, Chlosta’s running suture, was described [17, 18]. The aim of the current study was to compare complication rates and urinary function outcomes of patients who underwent LRP in terms of the VUA suturing technique in a single tertiary care center in Poland.
Aim
To compare three different types of sutures (Chlosta’s running suture, Van Velthoven single-knot running suture, V-Loc suture) that are used for VUA in LRP in terms of complication rates and urinary continence recovery.
Material and methods
Patients who underwent LRP between February 2014 and October 2018 in a single tertiary care center in Poland were enrolled in this retrospective study. The exclusion criteria were as follows: patients with a history of radiotherapy for PCa or distant metastases; preoperative stress-, urge, or mixed urinary incontinence; or a history of urethral trauma or stricture. Also excluded were patients with previous pelvic surgery, which might have affected their voiding function.
The study was approved by the local ethics committee. All operations were performed by four experienced surgeons, all of whom had performed more than 100 LRPs prior to the study. To evaluate the potential issue of operator bias in our study, we performed interaction tests between the operating surgeon and the effect of sutures on suturing time and the occurrence of complications.
Preoperative and perioperative data were extracted from medical records. Patients’ urinary continence status after catheter removal was assessed at 3, 6, 12, and 18 months after LRP. Urinary continence recovery was defined as the use of zero pads. The incidence of BNC was assessed concomitantly with urinary continence status up to 18 months after LRP. Complications were assessed within a 90-day postoperative period in accordance with the Clavien-Dindo classification [19].
Surgical techniques
Group 1: Chlosta’s running suture
After the urethra was cut off and full hemostasis achieved, VUA was performed with a single running suture using the center’s own modification. This method involved the use of a continuous suture (polyglactin 2-0, absorbable synthetic suture), which was placed first at the 5 o’clock position on the bladder neck outside-in and then inside-out toward the urethra, and the suture was tied. The continuous suture was then continued counterclockwise on the bladder neck outside-in and inside-out on the urethra and a tight anastomosis performed so that it covered the full thickness of the bladder wall and urethra. On average, five to seven needle passes were placed on both the bladder neck and the urethra until the free end of the suture at 5 o’clock was met. Just before the final suture of the anastomosis (Figure 1, Photo 1 G), an 18F silicone Foley catheter was introduced into the bladder under the surgeon’s direct vision. After both ends of the suture were tied, the watertightness of the anastomosis was assessed by filling the bladder with 100–200 cm3 of sterile physiological saline. If necessary, an additional suture was placed to avoid urinary leakage. After completion of the anastomosis, the balloon of the catheter was filled with 10 ml of sterile water. A 20F Redon drain was placed. In all patients with drainage output over 100 cm3 during the first 8 h after surgery, the creatine level of the fluid was assessed. The decision on catheter removal was at the discretion of the surgeon.
Group 2: Van Velthoven suture
After laparoscopic removal of the prostate, the bladder neck was identified. The running suture was prepared extracorporeally by tying together the ends of two 2-0 polyglycolic sutures, one dyed and one not, for identification purposes. The running suture was initiated by placing both needles outside-in through the bladder neck and inside-out on the urethra, one needle at the 5:30-o’clock position and the other at the 6:30-o’clock position. After three additional needle passes (bladder neck-urethra-bladder neck) symmetrically, counterclockwise with one thread and clockwise with the other, gentle traction was applied on each thread. At this point, an 18F silicone catheter was placed in the bladder. The suture was then continued until both threads reached the 12 o’clock position, outside-in on the bladder neck and inside-out on the urethra. To avoid tying the knot on the urethral side, the surgeon placed the last needle pass on either thread outside-in on the urethra and then inside-out on the bladder neck, after which the suture was tied. If maladjustment persisted between the diameters of the urethra and the bladder neck, the remaining anterior opening of the bladder neck was closed with 2-0 polyglycolic sutures (Photo 2). After the VUA was completed, the watertightness of anastomosis was assessed by filling the bladder with 100–200 cm3 of sterile physiological saline. The balloon of the catheter was filled with 10 ml of sterile water. A 20F Redon drain was placed. In all patients with drainage output over 100 cm3 during the first 8 h after surgery, the creatinine level of the fluid was assessed. The decision on catheter removal was at the discretion of the surgeon.
Group 3: Single barbed suture (V-Loc)
During VUA, a loop at the end of the V-Loc suture was used to anchor it to the neck of the bladder without the need for a knot. Moreover, while using this suture, the tissues of the bladder neck and urethra were opposed and secured in position after each needle passage, reducing the need for repeated suture tightening and tissue traction. The VUA was started on the bladder neck in the 5 o’clock position outside-in, and then the needle was passed through the urethra inside-out. A second needle pass was placed back on the bladder neck at the 6 o’clock position and from there it ran counterclockwise through the urethra and bladder neck. After a third pass with the needle through the bladder neck, gentle thread traction was applied to bring the bladder and urethra together. At this stage, an 18F Foley catheter was placed in the bladder. The entire anastomosis was performed with five to seven needle passes on both the urethra and the bladder neck. After finishing the VUA, the surgeon assessed the watertightness of anastomosis by filling the bladder with 100–200 cm3 of sterile physiological saline (Photo 3). The balloon of the catheter was filled with 10 ml of sterile water. A 20F Redon drain was placed. In all patients with drainage output over 100 cm3 during the first 8 h after surgery, the creatinine level of the fluid was assessed. The decision on catheter removal was at the discretion of the surgeon.
Statistical analysis
No sample size calculations were performed before initiating the study. Descriptive statistics on baseline variables are presented as median (interquartile range (IQR)) or count and percentage. Between-group differences were investigated using the Kruskal-Wallis rank sum test, χ2 test, or Fisher’s exact test as appropriate. A two-sided α level of 0.05 was used as a cutoff for statistical significance. Continuous variables were analyzed without any arbitrary categorization.
The association between the time of anastomosis and the suturing technique was investigated using a multiple linear regression model, with the type of suture and log-transformed size of the prostate in grams as predictors and the anastomosis time as the dependent variable. Potential violations of the model’s assumptions were inspected using diagnostic plots of the distribution of residuals.
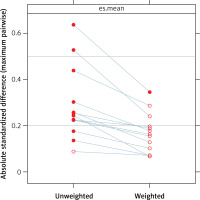
A doubly robust estimation procedure was used with a simultaneous propensity score weighting and covariate adjustment in a logistic regression model to estimate the average treatment effect of each suture type on the occurrence of complications up to 90 days after surgery. Under the assumption of exchangeability, this method answered the question about the average relative effects of all three suturing techniques in the population after we controlled for pretreatment imbalances on observed variables. Generalized boosted models with 30 000 trees were used for estimating propensity scores with the following variables: age, body mass index (BMI), clinical stage of the disease, Gleason grade (5 Grade Group system), log-transformed value of the prostate-specific antigen measurement, and size of the prostate in grams. Balance across treatment groups was checked visually with standardized effect size plots. Lingering imbalances between the groups were accounted for by using direct covariate adjustment in the final multiple logistic regression model.
The difference in time to recovery of urinary continence up to 1 year after surgery between the groups was studied using restricted mean survival time with covariate adjustment to control for potential confounding. We compared the mean time to recovery of urinary continence between Chlosta’s running suture, the Van Velthoven suture, and the V-Loc after adjusting for age, BMI, and size of the prostate. All analyses were performed with R version 3.6.2.
Results
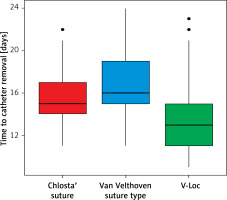
A total of 504 consecutive patients who underwent LRP for PCa between February 2014 and October 2018 in a single tertiary care center were enrolled in the study. The median age was 63 years (IQR: 58–68), the median BMI was 27 (IQR: 25–30), and the average UCSF-Capra (University of California, San Francisco, Cancer of the Prostate Risk Assessment) score was 3 (IQR: 2–5). Of these patients, 109 received Chlosta’s running suture, 117 the Van Velthoven suture, and 278 the V-Loc suture. Characteristics of the groups are presented in Table I. There were differences between groups regarding age, comorbidities (i.e., Charlson and American Society of Anesthesiologists score), clinical stage of the disease, Biopsy International Society of Urological Pathology (ISUP) score, and prostate volumes. Perioperatively, the groups were significantly different concerning time to catheter removal, intraoperative leaks, and percentage of clinically significant anastomotic leakage. During follow-up, differences were observed between groups for percentages of patients who developed BNC (Table II).
Table I
Baseline patient characteristics
[i] IQR – interquartile range, preop. PSA – preoperative prostate-specific antigen, BMI – body mass index, ASA – American Society of Anesthesiology, UCSF – Capra score – University of California, San Francisco, Cancer of the Prostate Risk Assessment, TRUS – transrectal ultrasound, ISUP – International Society of Urological Pathology.
Table II
Intraoperative, perioperative, and postoperative characteristics
The median time of anastomosis and the median time of surgery were 13 (IQR: 10–16) min and 150 (IQR: 120–220) min, respectively, in the Chlosta’s running suture group; 28 (IQR: 24–30) min and 175 (IQR: 150–205) min, respectively, in the Van-Velthoven suture group; and 12 (IQR: 11–16) min and 140 (IQR: 115–180) min, respectively, in the V-Loc suture group (p < 0.001) (Figure 2, Table II).
Figure 2
Time of anastomosis with different suturing techniques. Tukey’s boxplots with vertical black lines indicating median values
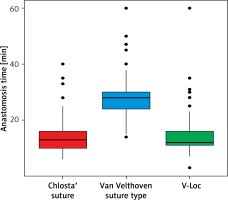
After adjustment for the size of the prostate after surgery in a linear regression model with a log-transformed dependent variable, the estimated time of anastomosis with the Van Velthoven suture was 105.6% longer (95% confidence interval (CI): 88.1–124.7) than that required for completing the procedure using either Chlosta’s running suture or the V-Loc. We did not find sufficient evidence to claim that the time of anastomosis was different when using V-Loc than it was when using Chlosta’s running suture, with a point estimate indicating a 1.7% prolongation of the procedure with V-Loc (95% CI: –5.7% to 9.7%), and we found no evidence for operator bias in interaction tests (all ps > 0.05).
Complications up to 90 days after surgery occurred in 20 (18.3%) patients in the Chlosta’s running suture group, in 27 (23.1%) patients in the Van Velthoven group, and in 37 (13.3%) patients in the V-Loc group (p = 0.051) (Table II).
Generalized boosted models were used to estimate the propensity score weights for receiving one of the three sutures for every patient. After confirming convergence of each of the three model fits, we visually checked the balance between groups (Photo 1, Figure 1). The average treatment effect of the suturing technique on the occurrence of complications, up to 90 days after the procedure, was estimated with a multiple logistic regression model weighted by the propensity score by using doubly robust estimation. The model summary is presented in Table III in the form of coefficients and corresponding standard errors. Estimated effects of interest, namely, the odds ratios for complications, were 1.65 (95% CI: 0.76–3.61) for the Van Velthoven suture and 0.90 (95% CI: 0.44–1.81) for the V-Loc suture compared with Chlosta’s running suture.
Table III
Doubly robust estimates from a weighted multiple logistic regression model
In the final pathology results, there were differences regarding the final ISUP score and lymph node metastases (Table IV).
Table IV
Pathological characteristics
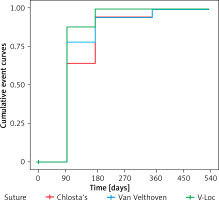
Unadjusted comparisons of the percentage of patients achieving urinary continence between groups are shown in Table V. Time to recovery of urinary continence after catheter removal (Figure 3), stratified by suture type, is shown in Figure 4. Data on urinary continence were missing in 24 patients who received the V-Loc suture. After we adjusted for age, BMI, and prostate size, the time to urinary continence recovery was on average 19 days shorter (95% CI: 5–33) when the V-Loc suture was used and 31 days shorter (95% CI: 16–45) during 1 year of observation when the V-Loc suture was used than when the Van-Velthoven suture or Chlosta’s running suture was used.
Table V
Urinary continence outcomes
Discussion
This study did not show significant differences in the percentages of continent patients between three different VUA type groups at 12 and 18 months after surgery. The results were comparable with previously published data [20], which suggests that regardless of the applied VUA technique, the patients’ long-term functional outcomes for urinary continence were satisfactory. However, the V-Loc group had faster continence recovery. Similar results were obtained in a small prospective study in patients with PCa who underwent robot-assisted radical prostatectomy [21]. Patients in the barbed suture VUA group had faster continence recovery in comparison with those in the classic Van Velthoven VUA group [21]. Interestingly, the difference in percentages of continent patients between VUA groups was also transient. According to that systematic review [21], the incidence of urinary leakage after LRP ranged from 3.2% to 33%. In the current study, the percentage of clinically significant urinary leakage was comparable in all VUA groups and did not exceed 2% for the entire cohort. However, definitions of urinary leakage differ between studies [22, 23] and the percentage of urinary leakage in the current study might be underestimated, as it was limited to patients with a drainage output of over 100 ml and an elevated creatinine level in the drained fluid.
In the Van Velthoven group, anastomosis time was longer than in the other two VUA groups, as was operating time. This might be a result of the more challenging technique of the Van Velthoven VUA and the requirement for simultaneous control of two threads. We found no significant differences between groups concerning complications up to 90 days after LRP, which is consistent with previous findings [24].
Data concerning the incidence of BNC after LRP are limited, but published reports show comparable results to those of our study [25–30]. In the Van Velthoven group, the incidence of BNC was high. This is a novel finding and no published data show similar results. A possible explanation is the increased tension in the Van Velthoven VUA or the greater number of needle passes, both of which might be linked with BNC [31–35].
In this study, the majority of patients underwent V-Loc VUA and the patients in that group had relatively unfavorable clinical risk factors and preoperative disease characteristics. The VUA type was chosen in accordance with the surgeon’s preferences, which might suggest that in more complicated cases, surgeons felt more comfortable choosing V-Loc VUA. The decision on time to catheter removal was also made by the surgeon. Catheterization time was shorter in the V-Loc group, which might suggest that surgeons felt more confident about the V-Loc VUA.
This study had several limitations. First, the estimated effects of suture types on the occurrence of complications could be viewed as causal only if the assumption of exchangeability (i.e., no unobserved confounding) was held; this warrants further investigation in an experimental setting. Second, with the sample size of this study, we were not able to rule out clinically relevant harm or benefit from using the studied suturing techniques because of the wide confidence intervals of the estimated treatment effects. Third, more sophisticated statistical modeling techniques such as restricted cubic splines or polynomials and the use of bootstrapping could have improved the quality of our inference even further. Fourth, this was a single-center study, which may limit the generalizability of our results.
The strength of this study was the consecutive sampling, which should provide adequate representativeness of the target population. We used doubly robust estimation, which protected against potential misspecification of either the propensity score model or the multiple logistic regression model. Finally, this was a pragmatic study that aimed to answer a clinically important question about the relative effects of three commonly used suturing techniques.