Purpose
Adenoid cystic carcinoma (ACC) of the base of the tongue (BOT) is extremely rare, with only 8.8% of ACCs occurring in the head and neck region [1, 2]. ACC is generally recognized by its typical characteristics of perineural invasion, indolent growth, late-onset local recurrence, and frequent distant metastases [3, 4]. The survival time of patients with early-stage ACCs is quite long due to slow growth pattern, but the long-term prognosis is relatively poor [5].
Radical resection with a negative margin combined with post-operative radiotherapy (RT) is the recommended treatment for ACC [6, 7]. Because of high propensity for ACC infiltration into adjacent tissues, it is difficult to ensure a pathological negative margin, even with a wide resection [8]. Patients with locally advanced neoplasms located on the BOT, often undergo glossectomy and partial or total laryngectomy, which causes considerable swelling and speech function defects [9].
The outcome of locally advanced ACC is poor, with 3-year progress-free survival (PFS) and overall survival (OS) rates of 44-48% and 60-72%, respectively [10, 11]. Chemotherapy, targeted therapy, and immunotherapy are used for patients with progressive and metastatic disease [12, 13], but objective responses are relatively low. Radiotherapy is one strategy for locally advanced tongue cancer, and it has the advantage of preserving tongue function compared to surgery combined with radiotherapy [14].
As a form of radiotherapy, iodine-125 (125I) interstitial brachytherapy (IBT) is used for the management of malignant neoplasms that occur in the salivary glands, especially locally advanced cases, with the advantage of organ preservation [15-17]. The present study retrospectively determined the efficacy and outcome of 125I IBT in the treatment of locally advanced ACC of the BOT.
Material and methods
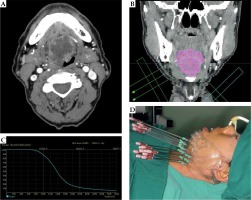
This was a retrospective study that was performed in accordance with international ethical standards and approved by the institutional review board. Nineteen patients who were pathologically diagnosed with ACC of the BOT treated with 125I interstitial brachytherapy were enrolled between March 2008 and April 2018. The median age was 56 years (range, 38 to 88 years). All patients (100.0%) had primary tumors, and no previous treatment was performed. The most common major complaint was pain (16/19, 84.2%). Other symptoms included tongue movement limitation (9/19, 47.4%) and tongue numbness (5/19, 26.3%). According to the TNM 8th staging edition of American Joint Committee on Cancer, all patients had stage T4aN0M0 disease. Table 1 shows the baseline characteristics of included patients. Surgery could not be performed in any patient due to local and systematic factors. Patients with distant metastases at diagnosis were excluded. All included patients provided written informed consent to receive 125I IBT, and agreed sharing their clinical data. None of the patients received external beam radiotherapy or chemotherapy. Half-life of 125I radioactive seeds was 59.4 days, and surface radioactivity was 18.5 to 25.9 MBq per seed. Length and diameter were 45 mm and 0.8 mm, respectively (model 6711, Beijing Atom and High Technique Industries Inc., Beijing, China). Gross tumor volume (GTV) was evaluated using computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET)/CT before treatment. Treatment plan was designed using DICOM format (treatment planning system, TPS, Beijing Atom and High Technique Industries Inc., Beijing, China), whereas clinical tumor volume (CTV) was defined as a 0-1.5 cm margin outside the tumor (the location where the tumor invaded the surface of the BOT, or the surface of the pharynx was defined as 0 cm) [18, 19]. Target volume was delineated and the prescribed dose ranged from 100 to 120 Gy in each patient. Organs at risk were then defined and included the parotid gland, submandibular gland, and skin, with a distance between organs at risk and D90 isometric line of more than 1 cm. Distribution of radioactive seeds was designed using TPS, and a three-dimensional (3D)-printed individual-puncture-guided template was designed. The needles were percutaneously punctured with sub-mandibular and sub-mental approaches. A 3D-printed individual template or CT navigation were used for implanting of 125I radioactive seeds under general anesthesia (Figure 1).
Table 1
Clinical characteristics of the 19 patients before 125I IBT for adenoid cystic carcinoma (ACC) of the base of the tongue
Fig. 1
A) Computed tomography (CT) image of a patient with adenoid cystic carcinoma (ACC) of the base of tongue (BOT); B) Clinical tumor volume (CTV) and the design of 125I radioactive seeds using TPS; C) Dose-volume histogram of CTV coverage (DVH) verification; D) 3D-printed individual template used during 125I IBT
Computed tomography examination was performed within one week after the treatment to verify the number, position, and dosimetry parameters.
Regular follow-up was required, with a CT examination of the head and neck region performed every 6 months, or whenever necessary. MRI or PET/CT was done for further examination if required, and chest radiography was performed once annually. Tumor regression, local relapse, and distant metastasis were evaluated from clinical and imaging findings. Tumor regression was evaluated according to response evaluation criteria in solid tumors (RECIST), and complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD) were assessed. RT-associated toxicities were evaluated using the Radiation Therapy Oncology Group (RTOG) grading system. Probability of local recurrence, OS and DFS were calculated using Kaplan-Meier method, and associations between outcomes were determined with log-rank test using SPSS v.20.0 software. Statistical significance was defined as a p-value less than 0.05.
Results
All patients successfully received 125I IBT treatment. The median number of 125I seeds was 71 (range, 37-124 seeds), and the median number of needles was 19. For CT vitrification during the first week, the mean D90 was 116.8 ±8.5 Gy, which was higher than the prescribed dose in all patients. The mean V100 was 92.0 ±1.3%, and was greater than 90% in all cases. In addition, for all patients, V150 was lower than 50%, and V200 was lower than 30%. The homogeneity index (HI) ranged from 20% to 50%, with the dose to organs at risk below the maximum limit.
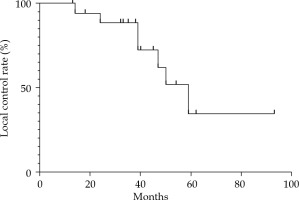
Six months after the treatment, all patients had local remission, and swelling and speech functions improved to different degrees. The median follow-up time was 35.0 months (range, 13-98 months). At 6 months, 7 patients (36.8%) had PR, 12 patients (63.2%) had a CR, and no patients had SD or PD (Figure 2). Seven patients (36.8%) experienced local recurrence, with an average recurrence time of 37.8 months (range, 13.5-58.5 months) since 125I IBT. Local failure occurred in 2 of these 7 patients within 2 years after the treatment (at 14 months and 24 months, respectively), and the other 5 patients had recurrent lesions after 3 years. Four patients did not receive treatment when local recurrence was detected due to old age, large size of the tumor, or the detection of brain metastases. Two patients received 125I IBT re-treatment, and 1 patient underwent surgery. The 3- and 5-year local control (LC) rates calculated by Kaplan-Meier method were 88.5% and 34.5%, respectively (Figure 3). The log-rank test revealed no significant differences in LC rate, based on age, sex, tumor size, distant metastasis, and matched peripheral dose.
Fig. 2
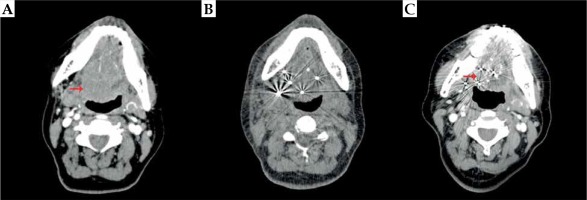
A) Pre-operative computed tomography (CT) examination of a patient with locally advanced adenoid cystic carcinoma (ACC) of the base of tongue (BOT); B) Verified CT examination one day after 125I IBT; C) Complete response three years after 125I IBT
Eight of the 19 patients died of the disease during follow-up period. Three patients (15.8%) died of local recurrence at 22, 50, and 59 months after 125I IBT treatment (Table 2). Two patients (10.5%) died of brain and lung metastases, and 2 patients (10.5%) died of lung metastases. One patient died of hemorrhage after 18 months, with no local recurrence or distant metastasis detected. Another patient died from other causes. The 3- and 5-year OS rates were 71.5% and 47.6%, respectively.
Table 2
Summary of treatment and outcomes for the 19 patients with adenoid cystic carcinoma (ACC) of the base of tongue (BOT)
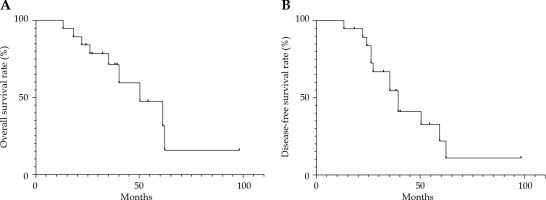
Neck lymph node metastases occurred in 2 patients (10.5%), and 8 patients (42.1%) had distant metastases (6 lung metastases, and 2 brain and lung metastases). The 3- and 5-year DFS rates were 54.7% and 21.9%, respectively (Figure 4).
Fig. 4
A) Kaplan-Meier curve for overall survival (OS) rate; B) Kaplan-Meier curve for disease-free survival (DFS) rate
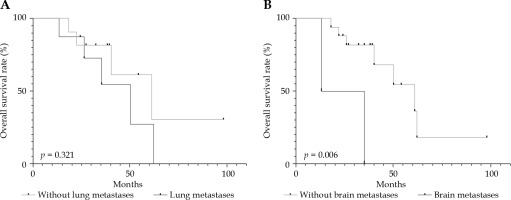
For the 3-year OS rates in patients with distant metastases (54.7%) and patients without it (81.8%), a p-value of 0.321 was calculated using log-rank test, and no significant difference was observed. Two patients with brain metastases died at 13 and 47 months, which was a significant difference compared to the OS of other patients (p = 0.006, < 0.5) (Figure 5).
Fig. 5
Overall survival rate according to (A) lung metastases and (B) brain metastases using Kaplan-Meier analyses
All patients underwent 125I IBT successfully under general anesthesia. Tracheotomy was performed in 1 patient because of swelling of the BOT and the floor of the mouth. The other patients were able to breathe freely, and no serious complications, such as airway obstruction, were noted. Minor acute toxicities (RTOG grades 1 to 2) were observed in all patients, including mucositis (n = 12 patients, 63.2%), erythema or dry desquamation (n = 10 patients, 52.6%), and mild dysphagia or odynophagia (n = 5 patients, 26.3%). No grade 3-4 acute toxicities were observed. One patient (5.3%) developed severe late toxicity. This patient had a hemorrhage 18 months after 125I IBT, and died due to airway obstruction, with no signs of local recurrence or distant metastasis. No other severe late toxicities (grades 3 to 4) were observed.
Discussion
Primary ACC of the BOT is rare. Unlike mobile tongue, early detection and diagnosis is difficult for ACC of the BOT because of asymptomatic growth pattern and posterior site [1, 20]. External beam radiotherapy (EBRT) plays an important role in the management of ACC. For early-stage cases, radical surgery combined with EBRT is the most common treatment. Mendenhall et al. concluded that OS rates of EBRT in ACC (5-year, 56%; 10-year, 43%) were lower than surgery combined with EBRT (5-year, 94%; 10-year, 91%) [21]. Recent studies reported that 5-year OS rates ranged from 59% to 74.8%, and 5-year LC rates ranged from 49% to 68.8% using proton radiotherapy (PRT) or carbon ion radiotherapy (CIRT) for head and neck ACC (HNACC) [10, 22-25].
However, the choice of surgery is controversial for locally advanced cases of ACC, especially of BOT. Quality of life decreased after wide surgical excision was performed [26]. As an alternative treatment, radiotherapy has advantages in function preservation. A 2009 German study reported 59 patients with inoperable or incompletely resected nasopharyngeal ACC, who were treated with bimodal RT consisting of intensity-modulated radiotherapy (IMRT) and carbon ion boost therapy (CIBT) [22]. Median follow-up time was 32 months, and 39 patients (67%) were alive at the last follow-up. LC, DFS, and OS rates at 5 years were 49%, 54%, and 69%, respectively. These outcomes (LC, DFS, and OS) were better in patients with GTVs larger than 100 cc than patients with GTVs smaller than 100 cc. There was a lower LC rate in patients with advanced disease (stage T4). The author concluded that adequate LC and OS rates were achieved using bimodal RT, and radiotoxicities were relatively mild. Another multi-center study in Japan included 289 inoperable patients, who were diagnosed with HNACC and treated with CIRT of 55.2 to 70.4 Gy [23]. 2-year OS, DFS, and LC rates were 94%, 68%, and 88%, respectively, and 5-year OS, DFS, and LC rates were 74%, 44%, and 68%, respectively. The authors suggested that good LC and mild toxicity were achieved for HNACC patients treated with CIRT. Also, a study by Koto et al. treated 18 patients who were diagnosed with ACC of the BOT (including 17 advanced cases with stage T4b) using CIRT [27]. Reliable efficacy was achieved, with 5-year LC, DFS, and OS rates of 92%, 44%, and 72%, respectively (Table 3). Compared to IMRT, the use of PRT and CIRT was limited due to equipment availability, and patients had to bear the financial burden.
Table 3
Summary of previous studies on locally advanced HNACC with radiotherapy
| Study [Ref.] | Year | Sites | No. of patients | Median follow-up (months) | Treatment | OS | LC | Factors influencing OS or LC |
|---|---|---|---|---|---|---|---|---|
| Akbaba et al. [22] | 2019 | Nasopharynx | 59 | 32 | IMRT with CIRT boost (72% for definite RT) | 2 years, 87% 5 years, 69% | 2 years, 83% 5 years, 49% | T4, GTV |
| Sulaiman et al. [23] | 2018 | All | 289 | 30 | CIRT | 2 years, 94% 5 years, 74% | 2 years, 88% 5 years, 68% | T stage, GTV, performance status |
| Ikawa et al. [24] | 2017 | All | 100 | 57 | CIRT | 5 years, 74.8% | 5 years, 68.6% | Prescribed dose, GTV, solid pattern |
| Gentile et al. [36] | 2017 | Base of skull | 14 | 69 | PRT | 5 years, 59% | – | – |
| Koto et al. [27] | 2016 | Base of tongue | 18 | 57 | CIRT | 5 years, 72% | 5 years, 92% | GTV |
Brachytherapy is increasingly accepted as an alternative treatment for inoperable head and neck malignant neoplasms. High-dose brachytherapy and iridium-192 (192Ir) IBT were used for tongue squamous cell carcinoma [28-30]. To the best of our knowledge, no research on brachytherapy or 125I was reported in ACC of the BOT. 125I IBT offers the advantages of minimal invasiveness, highly conformed delivery of high radiation dose to the target area, and a long half-life period. 125I IBT may be helpful in patients with inoperable diseases or poor general condition. Xu et al. reported 32 patients with local advanced ACC, involving the skull base treated with 125I IBT alone [31]. 3-year OS and DFS rates were 62.6% and 46.4%, respectively, with no severe acute RT reactions observed. Another research reported satisfying effects in 38 patients, who had a local failure or local advanced HNACC treated with 125I IBT [17]. Iodine-125 radioactive seed IBT is an effective treatment for ACCs, which are locally advanced or endanger vital organs, with acceptable toxicity. The present study reported the 3-year LC, DFS, and OS rates of 88.5%, 54.7%, and 71.5%, respectively, and were similar to the outcomes of CIRT in the treatment of inoperable ACC. The 5-year LC, DFS, and OS rates in our study were 34.5%, 21.9%, and 47.6%, respectively, and were worse than the effect of PRT or CIRT [22-24, 27, 32]. However, due to low incidence of ACC of the BOT, more studies are needed to improve long-term efficiency of IBT, and comparisons between 125I IBT and external beam radiotherapy are required as well.
Local control failure is an important cause of death, and local recurrence rates ranged from 35% to 53% in patients with HNACC [33, 34]. Akbaba reported 2- and 5-year LC rates of 83% and 49%, respectively, for nasopharynx ACC treated with IMRT and CIBT [22]. In another study, 3- and 5-year LC rates were 100% in patients with ACC of the BOT treated with CIBT [27]. In our study, 7 patients were confirmed to have a recurrence, with the recurrence rate of 36.8%. Two of these patients experienced a recurrence within 2 years, and other patients experienced a recurrence 3 years after 125I IBT. The 3- and 5-year LC rates in our study were 88.8% and 36.0%, respectively. Among the patients, who developed a recurrence, four did not receive further treatment, one received salvage surgery, and two received 125I IBT re-treatment. These results suggest that 125I IBT is suitable for locally advanced ACC of the BOT, with acceptable LC and survival. Iodine-125 IBT may also be used as a re-treatment in patients with recurrent lesions.
However, the improper handling of tumors of the BOT may lead to severe complications, including airway obstruction. EBRT may produce acute or late radiation-induced toxicities, and severe complications may occur, such as osteoradionecrosis and soft tissue necrosis [27, 35]. Koto et al. reported 2 occurrences of osteonecrosis of the jaw and 1 occurrence of hemorrhage among 18 patients with ACC of the BOT [27]. In the current study, 125I IBT was performed as a one-time procedure, in contrast to external beam radiotherapy. Relatively mild complications occurred in the patients who received 125I IBT compared to those who received CIBT or IMRT. No acute airway obstruction was observed while using the precisely designed individual templates and CT guidance. During the follow-up period, one patient experienced hemorrhage and died at 18 months, with no sign of local recurrence or distant metastasis. The hemorrhage may have been due to an extensive original tumor invasion and large target area. No severe complications were observed in other patients. Our results suggest that 125I IBT is minimally invasive and relatively safe for the treatment of ACC of the BOT. We should focus on respiratory conditions when GTV is relatively large.
Overall survival rate is significantly influenced by distant metastases, and 70% of patients’ deaths due to ACC were caused by lung metastases [8]. In our study, the 3-year OS rates of patients with and without lung metastases were 54.7% and 81.8% (p > 0.05), respectively. However, two patients with brain metastases died at 13 and 35 months, and brain metastases significantly influenced the OS rate (p = 0.032).
Our study has limitations because the sample size was relatively small. Firstly, the clinico-pathological characteristics and statistical analysis partially reflect this objective fact. Secondly, our follow-up period was relatively short. To our knowledge, ACC may recur after decades. Thirdly, in several patients, GTV was defined by multiple imaging, which could lead to inconsistency in the delineation of the target area. Further studies of 125I IBT efficiency and toxicity, with longer follow-up periods are needed.
Conclusions
In conclusion, 125I IBT is an option for the treatment of ACC of the base of the tongue, and provides relatively satisfactory LC and OS rates, with acceptable toxicities. Iodine-125 IBT is minimally invasive, conformal, and to some degree, prevents severe complications caused by surgery or EBRT. However, delineation of the tumor target area and matched peripheral dose demand further studies.