Summary
There is a need for direct indicators of atherosclerotic process slowing or growth. Baseline carotid intima-media thickness (CIMT) is independently associated with extent of atherosclerosis and ischemic cardiovascular events. Little is known about whether CIMT change may address atherosclerosis growth in individual patients, and as such whether CIMT change may be related to risk of major adverse cerebral and coronary event (MACCE) risk. Therefore, the present study aimed to evaluate the potential associations between CIMT change and the incidence of MACCE. Repeated assessment of CIMT changes might serve as a reflector of achieved atherosclerosis control during follow-up as it predicts the risk of major adverse cerebral and coronary event, recurrent myocardial infarction, and coronary disease progression.
Introduction
Despite percutaneous coronary intervention (PCI), cardiac rehabilitation and best medical therapy (BMT), survivors of first acute myocardial infarction (MI) are at high risk of a recurrent MI, or a subsequent vascular event and cardiovascular death (CVD) [1–3].
Most studies of post-MI outcomes focus on the acute phase after the index event, with few data available for follow-up beyond the first year. Although the risk of cardiovascular events is highest in the first year after the index MI, it remains remarkable in subsequent years [4]. The relative risk for all-cause death and cardiovascular outcomes (recurrent MI, CVD) is at least 30% higher than that in a general reference population at both 1–3 years and 3–5 years after MI [5]. It is true particularly for patients with additional risk factors such as diabetes, hypertension, or older age.
Furthermore, patients despite prescribed medications often demonstrate poor compliance, or the dosages prescribed are generally 50% or less of the dosages used in clinical trials [6, 7]. Both medication adherence and therapeutic goals are achieved in a minority of patients [6–8].
The data reinforce the importance of secondary prevention in improving long-term prognosis of post-MI patients. However, only a few, but indirect, prognostic tools are available, for instance assessment of the left ventricle function, biochemical markers of heart failure (e.g. NT-pro-BNP) or inflammatory process activity (e.g. hs-CRP), and occlusive stenotic lesions in extra-coronary vascular beds [9–12].
Yet, there is a need for direct indicators of atherosclerotic process slowing or growth, independent of prescribed pharmacotherapy and patients’ compliance. A potential indicator of cardiovascular risk might be carotid intima-media thickness (CIMT). Although baseline CIMT is independently associated with the extent of atherosclerosis and ischemic cardiovascular events [13, 14], little is known about whether CIMT change may address atherosclerosis growth in other than carotid artery territory in individual patients, and as such whether CIMT change may be related to risk of major adverse cerebral and coronary events (MACCE), recurrent MI or recurrent angina.
Aim
Therefore, the present study aimed to evaluate the potential associations between CIMT change and the incidence of MACCE, recurrent MI and new angina due to coronary lesion progression (CLP) in patients who underwent MI.
Material and methods
This prospective study comprised 215 patients (154 male; mean age: 63.3 ±9.8 years) admitted with recent MI, in whom PCI was performed for an index lesion. Of note, patients were treated according to contemporary guidelines concerning coronary intervention as well as best medical treatment (BMT) afterwards.
The CIMT assessments at baseline and during at least 2 follow-up visits were performed in all patients. The incidences of MACCE, CVD, recurrent MI, ischemic stroke (IS) and new onset angina were recorded prospectively for a median of 60 months.
Inclusion criteria were as follows: age over 18 years, acute MI with confirmed significant (at least 50% lumen reduction) stenosis in an index coronary artery.
Exclusion criteria: chronic or acute inflammatory status, active cancer, critical state, acute heart failure or with congestive heart failure in NYHA classes IV, patients not willing to participate in the present study, or suboptimal carotid artery ultrasound (e.g. massive calcifications enabling CIMT assessment).
According to current guidelines, patients were prescribed dual antiplatelet therapy and other medications, including typically a statin, β-blocker, and angiotensin converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB), as per guidelines of the European Society of Cardiology [15].
All subjects gave their informed consent prior to enrolment in accordance with the requirements of the institutional local Ethics Committee. The study was performed consistently with the requirements of the Declaration of Helsinki.
Carotid intima-media measurement
Carotid ultrasonography was done with high-resolution B-mode, color Doppler, and pulsed wave Doppler ultrasonography, using a Toshiba Aplio 300 ultrasound machine (Toshiba Medical Systems Co) equipped with a 4–12-MHz linear array transducer.
During hospital stay (baseline examination), all patients underwent measurement of the maximum carotid intima-media complex of the near and far walls at 3 segments, including the distal 1 cm of the common carotid artery, at the bifurcation, and at the level of the internal carotid artery. All measurements were expressed as mean maximum CIMT.
After initial CIMT assessment, participants were followed up twice with CIMT re-evaluation at a mean of 3 and 4.5 years.
The annual change in CIMT (mm/year) was calculated using the following equation: annual change of CIMT = (follow-up CIMT – baseline CIMT)/observation period in years.
The difference between the follow-up and baseline CIMT was also expressed as an absolute Δ value according to the following equation: ΔCIMT = follow-up CIMT – baseline CIMT.
Follow-up period and cardiovascular events
The clinical follow-up was continued beyond the last CIMT re-assessment in order to establish the potential relationship between CIMT change and MACCE, recurrent MI and new onset of angina for a median of 60 months.
Recurrent MI was diagnosed according to contemporary criteria of the European Society of Cardiology [16]. The diagnosis of IS was established by a neurologist to ensure validity. The CVD was defined as fatal IS, fatal MI, or other CVD (i.e., any sudden or unexpected death unless proven as non-cardiovascular on autopsy). The relationship between new onset angina and coronary status was established with coronary angiography in order to ensure coronary lesion progression, and exclude symptoms related to stenosis recurrence (restenosis or stent thrombosis as related to different mechanisms) [17, 18]. For further analysis only new angina due to CLP was taken into account.
Statistical analysis
Student’s t test was used for comparison of continuous variables, and the χ2 test was used to compare proportions of categorical variables. Means of the analyzed parameters across groups were tested by analysis of variance test. Frequencies were compared by the χ2 test for independence.
The influence of CIMT change, clinical, and biochemical factors on the incidence of the endpoints MACCE, recurrent MI, and new angina due to CLP was assessed with Cox univariate hazard analysis, and in cases with a p-value < 0.05, they were included in a multivariate stepwise Cox proportional hazards analysis. The results of the multivariate Cox proportional hazards analysis were expressed as hazard ratio (HR) and 95% confidence interval (CI). Kaplan-Meier analysis for recurrent MI and new angina due to coronary lesion progression according to quartiles (A – lowest; D – highest quartile) of the annual CIMT change on the final ultrasonography was performed.
Receiver operating characteristic (ROC) curves were constructed to establish the best cut-off value for the annual CIMT progression rate predicting risk of MACCE, recurrent MI and CLP. The area under the ROC curve (AUC) was calculated. Statistical analyses were performed with Statistica version 12.0 software (StatSoft, Inc, Tulsa, OK). P < 0.05 was considered statistically significant.
Results
During the median 60-month follow-up period, MACCE were recorded in 65 (30.2%) patients, including 10 CVDs (9 MIs/sudden cardiac, 1 IS), 42 non-fatal MIs and/or 15 non-fatal ISs. Recurrent MI occurred in 12 (5.6%) subjects during first year of follow-up, and in 26 (12.1%) at 3 years. Additionally, angina recurrence in CCS classes II–III was noted in 30 (14%) patients, of which new angina due to CLP was observed in 20 (9.3%) patients, while angina due to in-stent restenosis was observed in 10 (4.7%), as evidenced with coronary angiography.
The detailed clinical characteristics and assessment depending on MACCE plus CLP incidence are summarized in Table I. Subjects in whom MACCE plus CLP occurred, as compared to the MACCE-free subgroup, were more often hypertensive (p = 0.026), had higher levels of serum creatinine (p = 0.049) and hs-CRP (p = 0.001), and had a higher mean number of cardiovascular risk factors among hypertension, hyperlipidemia, diabetes, and cigarette smoking (p = 0.058) (Table I).
Table I
Baseline characteristics of the study group
Initial CIMT values were similar in patients who suffered MACCE and event-free patients (1.43 ±0.40 mm vs. 1.45 ±0.44 mm; p = 0.486).
At first CIMT reassessment during the mean follow-up period of 36.5 ±29.3 months, patients in whom MACCE occurred had 2.7-fold greater annual CIMT growth as compared to the event-free subset of patients (0.024 ±0.12 mm vs. 0.009 ±0.16 mm/year; p < 0.001). Similar findings concerned the final CIMT reassessment performed during the mean follow-up period of 53.3 ±37.1 months (0.050 ±0.1 mm vs. 0.001 ±0.1 mm/year; p < 0.001, respectively).
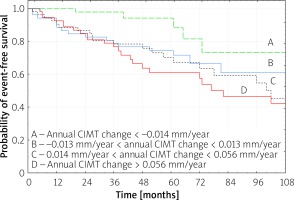
In patients in whom annual CIMT change showed clear carotid atherosclerosis reduction (1st quartile for annual CIMT change), a significantly lower number of recurrent MI and new angina due to CLP was observed, as compared to subjects with annual CIMT changes in the 2nd, 3rd and 4th quartiles (p = 0.008 by log rank test), and as compared to the 4th quartile (p < 0.001 by log rank test) (Figure 1). The Kaplan-Meier free-survival curves were 100, 97.8 and 88.4% at 1, 3 and 5 years for the 1st quartile, while they were 92.6, 76.6 and 61.0% for the 4th quartile, respectively (Figure 1).
Figure 1
Kaplan-Meier analysis for recurrent MI and new angina due to coronary lesion progression according to quartiles (A – lowest; D – highest quartile) of the annual CIMT change on the final ultrasonography (p < 0.001 for A vs. D by log-rank test; p = 0.008 for A vs. B, C, D by log-rank test)
Cox univariate regression analysis indicated several factors possibly associated with cardiovascular event risk, of which a multivariate stepwise Cox proportional hazards analysis identified that risk of MACCE was associated independently with the annual CIMT progression > 0.003 mm/year and serum creatinine level (Table II). Recurrent MI, as well as recurrent MI and new angina due to CLP, was associated with the annual CIMT progression > 0.003 mm/year, serum creatinine level, and history of hyperlipidaemia (Table II).
Table II
Multivariate stepwise Cox proportional hazards analysis. Independent risk factors of MACCE, recurrent MI and recurrent MI plus coronary lesion progression (CLP)
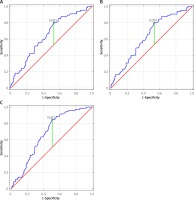
The CIMT change was found to be an independent indicator of MACCE, recurrent MI and MI plus CLP with an optimal cut-off value of CIMT progression rate of > 0.003 mm/year for all study end-points (Figures 2 A–C). Although the annual CIMT progression rate of 0.003 mm/year was the best predictor of MACCE, recurrent MI and CLP, associated with fairly good sensitivity (between 80% and 84.5%), it demonstrated only mild specificity, between 47% and 49.3% (AUC between 0.638 and 0.673) (Table III).
Table III
Receiver operating characteristic (ROC) curves for the optimal annual CIMT progression rate cut-off value to predict risk of MACCE, recurrent MI and CLP
| Event | Annual CIMT progression [mm/year] | AUC | 95% CI | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| MACCE | 0.003 | 0.651 | 0.574–0.728 | 80.3 | 47 |
| Recurrent MI | 0.003 | 0.683 | 0.561–0.716 | 80 | 47.6 |
| Recurrent MI plus CLP | 0.003 | 0.673 | 0.601–0.744 | 84.5 | 49.3 |
Discussion
In the first decade of the twenty-first century, in survivors of first MI, the cumulative incidence of death was 12.1% at 1 year and 17.5% at 3 years, while recurrent MI were 5% and 8.2%, respectively [3]. In the years between 2004 and 2010, in England, in all survivors of a first acute MI, the risk of a second MI was highest during the first year and the cumulative risk increased more gradually thereafter [1]. For men, 1- and 7-year cumulative risks were 5.6% and 13.9%; for women, they were 7.2% and 16.2% [1]. Data from Swedish national registries of 108 315 patients admitted to hospital with a primary MI between the years 2006 and 2011 demonstrated that the composite endpoint of non-fatal MI, non-fatal IS or CVD was 18.3% during the first year after the index MI [2].
In the present study, the 5-year MACCE incidence was 30.2%, and including CLP 38.6%. Recurrent MI occurred in 5.6% of subjects during the first year of follow-up, and in 12.1% at 3 years. Thus, despite the attempt to implement BMT according to guidelines, our data are consistent with others in terms of MACCE incidence.
Moreover, in patients who suffered from MACCE, we found a 2.7-fold greater annual CIMT progression rate as compared to the event-free subgroup of patients. Our study indicated that the annual CIMT progression rate of 0.003 mm/year is a sensitive marker of risk for MACCE, recurrent MI and CLP (sensitivity between 80% and 84.5%), but it demonstrated only mild specificity between 47% and 49.3% (AUC between 0.638 and 0.673).
In line with our findings, in the CLAS trial, patients with an annual common CIMT progression rate of 0.034 mm/year had a 2.9-fold higher CVD risk compared to those with a common CIMT progression rate of 0.011 mm/year or less [19].
Consistent with our results, Okayama et al. observed that a higher median progression rate of common CIMT (0.03 mm/year) was a significant determinant of MACCE (HR = 2.24; 95% CI: 1.25–4.03, p < 0.01) during a mean follow-up of 7.6 years [20]. Controversially, in the IMPROVE study, assessment of 15-month progression demonstrated that only the fastest CIMT max-progression was significantly associated with risk of subsequent vascular events [21].
We demonstrated that when patients were stratified into quartiles based on the annual change in CIMT, patients in the 1st quartile had a lower probability of recurrent MI and CLP, as compared to patients in the other quartiles. This finding is in line with a study of Hirano et al., including 240 patients with stable coronary artery disease [22]. Furthermore, Hirano et al. found that addition of the change in plaque-CIMT max in the follow-up period of 6 months, to conventional risk factors, resulted in a greater area under the ROC curve compared with conventional Framingham risk factors alone (0.81 and 0.70, respectively, p = 0.02) [22].
The idea of atherosclerosis tracking through serial CIMT assessments in patients with cardiovascular risk factors in the primary and the secondary prevention populations is very attractive as objective indicators of achieved atherosclerosis control would improve health outcome [23].
Many data report poor compliance and frequent underdosage of medications in patients discharged for MI. Korhonen et al. found that only 49% of the patients adhered (≥ 80% of days covered) to all 3 medications including ACE inhibitors/ARBs, β-blockers, and statins [24]. It is estimated that after 1, 3, and 5 years, 78, 64, and 58% of survivors who had started therapy were still receiving β-blockers, 86, 78, and 74% were receiving ACE-inhibitors, and 85, 80, and 82% were receiving statins, respectively [7]. Furthermore, the dosages prescribed were generally 50% or less of the dosages used in clinical trials, and dosages did not increase during the observation period [7]. Patients who did not start treatment shortly after discharge had a low probability of starting treatment later [7].
Moreover, 6 months after an MI, even in patients with a high adherence to drug treatments (≥ 90% of days covered), blood pressure < 140/90 mm Hg, low-density lipoprotein (LDL) < 100 mg/dl (in patients on statins), HbA1c < 7% (in treated diabetics), and smoking persistence were observed in 74%, 76%, 45%, and 27% of patients, respectively [25].
As we found in the present study, CIMT change over time might serve as an objective marker of atherosclerosis control, and as such, without much knowledge on patients’ drug adherence and achieved control of cardiovascular risk factors, might serve as an additional tool to encourage lifestyle modification and motivational education programs to reduce risk of cardiovascular events.