Introduction
Pancreatic adenocarcinoma accounts for approximately 3% of all malignant neoplasms, with a constantly increasing incidence. One of the most important risk factors for developing pancreatic cancer is age. It is estimated that in the USA just 13% of all pancreatic cancer cases occur in people under the age of 60 years. In Poland, in 2019, over 65% of pancreatic cancer diagnoses were in patients aged 65 years or over. 3621 patients over 64 years of age died due to pancreatic cancer, which accounts for over 70% of all deaths from this cancer [1].
The treatment of elderly patients is particularly challenging. This population is especially vulnerable to complications, which results, among others, from the coexistence of other diseases and related potential drug interactions after the use of cytostatics. According to research, as many as 80% of patients over 65 years of age diagnosed with pancreatic cancer are taking permanently at least 5 different medications due to comorbidities [2]. Moreover, in this age group, organ reserves are often reduced, including impaired renal function. The pharmacokinetics of drugs is also significantly altered. Due to reduced absorption capacity resulting from the atrophy of the gastrointestinal mucosa, the target serum concentration of oral drugs may be inadequate.
The metabolism of cytostatics is also reduced; this is due to, among others, decreased hepatic blood flow and cytochrome p450 activity. Decreased total body water, in combination with decreased serum albumin, lead to disturbances in drug distribution. Finally, reduced glomerular filtration affects the excretion of drugs from the body, which may contribute to increased toxicity of treatment [3].
The age of 65 years was generally adopted as the conventional elderly age limit. Because aging is a highly individualized process, the biological age of patients does not always correspond to their metrical age. In addition to genetic predisposition, environmental factors and lifestyle are also responsible for the aging of the body. Smoking, alcohol abuse, and a poor diet are not only conducive to faster aging but are also risk factors for the development of pancreatic cancer. Therefore, in the group of patients with this diagnosis, the expected percentage of patients who will tolerate systemic treatment significantly worse can be essential [4]. However, patients with diagnosed cancer aged 65 years and over still constitute a heterogeneous group, in which a subgroup of patients in good general condition with adequate organ capacity should also be distinguished, for whom, despite the metrical age, it is worth undertaking the same treatment as in the group of younger patients.
The world literature contains a small number of reports on the treatment of pancreatic cancer in the elderly population. In the registration study for the FOLFIRINOX protocol, the median age of the treated patients was 61 years, and the age of over 75 years was an exclusion factor from the study [5]. This single-centre retrospective study presents the results of treatment of advanced pancreatic adenocarcinoma in patients aged 65 years and over.
Material and methods
Data from 43 patients who were treated in the years 2017–2021 were analysed retrospectively. The 2 main inclusion criteria were advanced stage of the disease, defined as definitively inoperable or disseminated pancreatic cancer, and age – at least 65 years at the time of systemic treatment initiation. All patients were treated with a biweekly FOLFIRINOX regimen. The standard dose of the drugs was 85 mg/m2 of oxaliplatin given intravenously for 2 hours, 180 mg/m2 of irinotecan given intravenously for 1.5 hours, 200 mg/m2 of levofolinic acid given intravenously for 2 hours, 400 mg/m2 of 5-fluorouracil given intravenously as a bolus, and 2400 mg/m2 of 5-fluorouracil given intravenously in a 46-hour infusion. The treatment was carried out to progression or unacceptable toxicity, some with minor modifications of the therapy – the dose and schedule of chemotherapy was adjusted by clinical oncologists in response to the adverse events observed in the previous cycle. Toxicity was assessed based on the National Cancer Institute Common Toxicity Criteria scale, version 5.
The performed analysis included the occurrence and severity of chemotherapy complications as well as its effectiveness measured by the length of survival. The overall survival (OS) was defined as the time from the beginning of the treatment to the date of death from any cause. For participants without documentation of death, OS was censored on the last date the participant was known to be alive (last visit).
Progression free survival (PFS) was calculated from the date of starting treatment until the date of the progression of the disease confirmed in computed tomography. Survival rate estimates were calculated with the use of the Kaplan-Meier method. All statistical analyses were performed using Microsoft Excel and Statistica version 13.
Referring to the type of study, acting on the basis of Polish law and Good Clinical Practice, the consent of the Bioethics Committee was not required.
Results
Men accounted for 37% of the study population (16 patients) and women 63% (27 patients).
The median age of the treated patients was 68 years (range 65–79 years). Patients at least 70 years of age constituted 37% of patients (16 people).
The main demographic and clinical characteristics of the patients are presented in Table 1.
Table 1
Demographic and clinical characteristics of the patients
The median number of cycles administered was 10.5 (range 2–44), and the median follow-up was 9.1 months. In the study group, 41 patients (95%) finished the chemotherapy treatment according to the FOLFIRINOX regimen.
The reasons for terminating the therapy were:
neoplastic disease progression: 34 patients (83%),
side effects: 2 patients (4.9%),
loss of follow-up of 5 patients (12.1%).
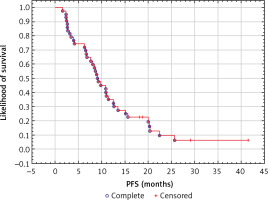
The median PFS in the entire study group was 8.8 months (Fig. 1). In the subgroup of patients with disseminated disease the median PFS was 7.6 months, and in the subgroup with locally advanced disease it was 10.9 months.
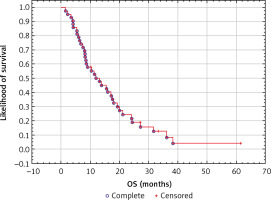
The median OS was 9.8 months (Fig. 2): in the disseminated disease subgroup it was 8.3 months, while in the subgroup with locally advanced disease it was 12.2 months.
In the study population, an adverse reaction was reported at least once in all patients, with 93% of them having at least 3 different treatment complications. In most cases (92% of all adverse events), their severity was rated 1 or 2 according to the common terminology criteria for adverse events [6]. Common terminology criteria for adverse events grade 4 complication was reported in only one patient and was related to neutropaenia. Due to side effects, dose reduction of cytotoxic drugs was required by 36 (83%) patients, while in 2 (5%) cases complications resulted in premature termination of therapy. In both, they were in the form of G3 polyneuropathy.
Haematological disorders were reported among the most common side effects. Anaemia was observed in 36 (84%) patients, and in 48% it was mild. Moderate and severe anaemia was found in 12 (28%) and 3 (7%) patients, respectively. Moreover, the weakness associated with systemic treatment, which often correlates with the comorbidity of anaemia, was reported by 35 (81%) patients, and it was mild in 25 (58%) cases and moderate in 10 (23%) cases.
The next most common complication was thrombocytopaenia, which occurred in 26 (60%) cases, but it was severe only in one (2%) patient.
Immunodeficiency as a neutropaenia was present in 23 (53%) patients, of which 9 (21%) caused the chemotherapy cycle delay (G2 disorder or greater). In a group of 37 (86%) patients receiving primary prophylaxis of febrile neutropaenia with granulocyte-colony stimulating factor (G-CSF), neutropaenia was observed in 18 (41%) cases, of which 5 (12%) caused the next cycle of chemotherapy delay. Due to neutropaenia occurring during treatment, it was necessary to re-introduce G-CSF in 3 (7%) patients.
Among the other most common adverse reactions, hepatic toxicity was mild in 28 (65%) patients, and moderate in 5 (12%). No severe, toxic liver damage was observed.
Gastrointestinal complaints affected about half of the patients. Moderate diarrhoea was observed slightly more frequently (mild in 17 (40%) patients, moderate in 2 (5%) patients, and severe in 4 (9%) patients) than vomiting, which was mild to moderate in 13 (31%) patients.
Polyneuropathy concerned a total of 22 (41%) patients, in 5 (12%) cases it was severe, and in 2 cases it was the cause of termination of chemotherapy.
Other less frequent complications include aphthous stomatitis [G1 in 4 (9%) patients and G2 in 1 (2%) patients], and mild hand-foot syndrome was observed in 2 (5%) patients.
Multi-morbidity, defined as the coexistence of at least 2 diseases in a patient, was found in 39 (90%) patients. The most common comorbidities included arterial hypertension in 24 (56%) and type 2 diabetes in 22 (51%) patients. Coronary heart disease concerned 8 (19%) patients. In the group of patients treated for arterial hypertension, a significantly lower risk of complications in the form of neutropaenia was observed (r Spearman –0.34; p = 0.02), while in the group of patients diagnosed with diabetes, nausea after chemotherapy was significantly less frequent (r Spearman – 0.33; p = 0.03). Moreover, the statistical analyses showed no statistically significant correlation between the coexistence of comorbidities and complications of systemic treatment (Table 2).
Table 2
Complications of systemic treatment in the study group depending on their severity
Discussion
In the study group of patients aged 65 years and over, treated with chemotherapy according to the FOLFIRINOX regimen for advanced pancreatic cancer, the median OS time was 9.8 months in patients with metastatic disease and 12.2 months in patients with locally advanced pancreatic cancer. These results are comparable to both the registration study for the FOLFIRINOX protocol [5] and the results from other literature reports [7, 8].
It is worth noting that similar results were presented in one of the studies on a small population of even older patients – patients over 74 years of age, where the median OS was 11.6 months [9].
In the case of the median time to progression of cancer, the results are also comparable with global data.
In the presented analysis, adverse events were described in almost all treated patients, but their severity was serious or very serious only in 8% of cases.
Grade G4 side effects were reported in one patient (2% of all complications), and it was neutropaenia without concomitant infectious symptoms. Overall, G3–G4 neutropaenia was reported in 7% of treated patients. This is much less than in the registration study (45.7%) and results from the use of granulocyte growth factors in the primary prophylaxis of febrile neutropaenia in this group of patients. Due to the risk factors for this complication, prophylaxis was used in almost all patients (84%). In the registration study, granulocyte growth factors were not recommended.
In literature reports, primary prophylaxis of neutropaenia in the elderly group was used in less than 40% of patients [10].
The correlation shown in the study between the reduced risk of neutropaenia in patients with additional treatment for hypertension is not entirely clear. Confirmation of such a relationship would require an analysis on a larger study population. Similar conclusions apply to the relationship observed in the study regarding the lower intensity of nausea in patients with diabetes.
Anaemia was reported in 82% of patients, but only 7% progressed to G3. This result is comparable to that of Conroy’s work [5].
Among the non-haematological side effects of chemotherapy, the most common were asthenia (81% of patients), diarrhoea (54%), and polyneuropathy (51%).
In the case of polyneuropathy, severe exacerbation of this complication (G3) occurred in 12% of patients; this is slightly more than in the registration study and in other literature reports [10, 11].
This result confirms that the elderly population is particularly vulnerable to developing polyneuropathy during systemic treatment.
Asthenia occurred in the majority of patients (81%); none of the patients had this complication worse than G3. In the literature, weakness was observed in 48–94% of patients, and G3 severity was observed in up to 10% of patients [11].
Among the gastrointestinal complications, the most clinically significant was diarrhoea, which occurred in more than half of the patients, and in whom 9% it was severe. In the available literature data, severe diarrhoea affected 7–25% of the treated population aged over 65 years.
In the study group, 83% of patients required reduction of cytostatic doses due to treatment toxicity. This is more than previously published – the largest percentage of patients who required a reduction in treatment intensity was 75% [11].
Taking into account the results of OS and PFS, dose reduction in this group of patients did not translate into worsening of treatment results, but it certainly improved the quality of life of the treated patients.
Conclusions
The presented results show that the age of the patient diagnosed with pancreatic cancer should not be a contraindication to the choice of a relatively aggressive treatment method, i.e. chemotherapy with the FOLFIRINOX regimen. Appropriate qualification of patients and careful supervision during treatment result in effectiveness comparable to the treatment of the younger population with acceptable intensity of therapy complications