Introduction
The aryl hydrocarbon receptor (AHR) is a ligand-dependent transcription factor that regulates gene expression in a range of cells, including immune and epithelial cells. The AHR signalling pathway plays an important role in various physiological processes, including the regulation of skin homeostasis, inflammation, and immune responses. It accomplishes this by regulating various aspects of skin function, including the immunological network, keratinocyte differentiation, skin barrier function, pigmentation, and responses to oxidative stress.
AHR signalling pathways and regulatory functions
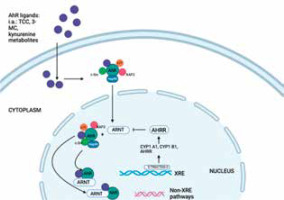
AHR is a ligand-activated transcription factor that belongs to the basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) family of proteins [1]. In the absence of its activating ligands, such as certain polycyclic aromatic hydrocarbons (PAHs), AHR is associated with a cytoplasmic protein complex that includes heat shock protein 90 (HSP90) and other chaperone proteins [2]. In the skin, AHR is primarily expressed in the epidermis, including keratinocytes and Langerhans cells.
By controlling responses to xenobiotic and environmental stressors, AHR is essential for preserving homeostasis. It is activated by a variety of low-molecular-weight ligands that can be obtained from endogenous, dietary, environmental, and microbial sources [3]. The endogenous ligands of the AHR encompass a range of compounds such as indigoids, heme metabolites, and arachidonic acid metabolites. Dietary AHR ligands include flavonoids, carotenoids, and metabolites produced by commensal gut bacteria. Additionally, environmental AHR ligands consist of PAHs and polychlorinated biphenyls. The varied ways in which AHR is activated by this diverse spectrum of ligands is an essential trait [4]. The AHR is a transcription factor that plays a crucial role in mediating the toxic effects of environmental pollutants and also participates in various physiological processes [5]. The AHR can be activated through canonical and non-canonical pathways. The canonical activation pathway of AHR is initiated by the binding of specific ligands, such as dioxins, PAHs, and certain synthetic compounds. These ligands are generally hydrophobic and lipophilic in nature. In the cytoplasm, AHR exists in an inactive state complexed with HSP90 and other chaperone proteins [6]. Upon binding to its ligand, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the AHR ligand-activated AHR undergoes a conformational change, which triggers its translocation into the nucleus [7–9]. Translocation into the nucleus is facilitated by several proteins, including the AHR nuclear translocator (ARNT). The activated AHR-ligand complex heterodimerizes with the ARNT, resulting in transformation of the complex into a high-affinity DNA-binding transcription factor. ARNT, also known as hypoxia-inducible factor 1β (HIF-1β), is constitutively present in the nucleus [10]. It contains a basic helix-loop-helix (bHLH) domain and a PAS domain, similar to AHR. Once in the nucleus, the activated AHR forms a heterodimeric complex with ARNT. The bHLH and PAS domains of AHR and ARNT interact with each other, stabilizing the complex. The complex formed by the AHR-ligand and ARNT interacts with specific DNA recognition sites, leading to the regulation of gene transcription for AHR-responsive genes. It plays a crucial role in the regulation of gene expression by binding to specific DNA sequences called xenobiotic response elements (XREs) or dioxin response elements (DREs) in the promoter regions of target genes. The binding of the AHR-ARNT complex to XREs recruits additional coactivator proteins and initiates the transcriptional activation of target genes [4]. The target genes regulated by AHR-ARNT are involved in various cellular processes, including xenobiotic metabolism, oxidative stress response, immune regulation, and cell proliferation. Examples of target genes include cytochrome P450 enzymes (CYP1A1, CYP1A2), NAD(P)H: quinone oxidoreductase (NQO1), and several cytokines and growth factors [11]. ARNT is involved in mediating the transcriptional activation or repression of various genes, both in conjunction with AHR and independently, regulating processes like drug metabolism, cell proliferation, and responses to hypoxia [12]. Besides its primary impact on gene transcription, AHR has the ability to activate other transcription factors, including nuclear factor κB and nuclear factor erythroid 2-related factor 2 (Nrf2), in order to regulate gene expression [13–15]. Consequently, the activation of AHR by various ligands can lead to the upregulation or downregulation of distinct genes, thereby eliciting a wide range of biological responses across several tissue types.
The non-canonical activation pathway of AHR is independent of the canonical ligand-mediated activation. In this pathway, AHR can be activated by various endogenous and exogenous stimuli, including certain metabolites, oxidative stress, inflammation, and altered cellular redox status. Unlike canonical activation, non-canonical activation does not involve ligand binding and nuclear translocation. Instead, non-canonical activation often occurs in the cytoplasm or at the plasma membrane. In response to stimuli, AHR can be modified by phosphorylation, acetylation, or ubiquitination, leading to changes in its conformation and activity [16]. Although the non-canonical pathway is less well-defined compared to the canonical pathway, it highlights the versatile and multifaceted nature of AHR signalling beyond the classical ligand-dependent activation.
It is important to note that the canonical and non-canonical pathways of AHR activation are not mutually exclusive, and they can often intersect and interact with each other to regulate gene expression and cellular responses in a context-dependent manner [10, 11] (Figure 1).
Figure 1
The AHR signalling pathway. The aryl hydrocarbon receptor (AHR) is a transcription factor that is activated by ligand binding. In its inactive state, AHR forms a complex with heat shock protein 90 (Hsp90), X-associated protein 2 (XAP2), c-Src, and p23 in the cytoplasm. Upon binding to the ligand, AHR undergoes conformational changes, leading to the formation of complexes with ARNT, which then translocate into the nucleus. The XRE is located in the genomic regulatory region of AHR target genes, and in addition to detecting the XRE in the nucleus, the AHR-ARNT complex also interacts with other transcriptional regulators to regulate the expression of several target genes. The AHR, or aryl hydrocarbon receptor, is a protein involved in the regulation of gene expression. It forms a complex with ARNT, or aryl hydrocarbon receptor nuclear transport protein, and binds to XRE, or exogenous response element, to activate the transcription of genes, including those encoding cytochrome P450 enzymes (CYP)
AHR is known to regulate the expression of a wide range of genes involved in various physiological processes, including xenobiotic metabolism, immune response, cell cycle regulation, and development. AHR-ARNT mechanism allows the cell to respond to environmental cues and regulate gene expression accordingly. Dysregulation of this pathway has been associated with various diseases, including cancer, immune disorders, and developmental abnormalities [17]. The AHR signalling pathway plays a significant role in dermatoses, which are a group of skin disorders characterized by various symptoms, including inflammation, itching, redness, and abnormal cell proliferation (Figure 2).
Figure 2
Epidermal cellular responses to prolonged activation of the aryl hydrocarbon receptor (AHR) due to environmental factors. Prolonged activation of the aryl hydrocarbon receptor (AHR) due to long-term exposure of the skin to either UVB radiation or chemical pollutants might impact several cellular processes that lead to external skin aging and the formation and advancement of skin cancer
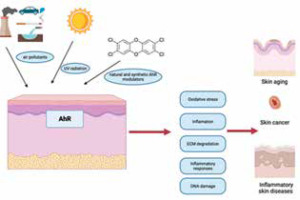
Activation of the AHR pathway occurs when ligands, including endogenous compounds or environmental pollutants, bind to the AHR protein. This triggers a cascade of events leading to the transcription of target genes involved in various cellular processes, including inflammation, immune response, and tissue repair [18]. AHR activation promotes the differentiation of keratinocytes and the formation of a functional skin barrier. It regulates the expression of genes involved in epidermal differentiation and cornification, such as filaggrin, loricrin, and involucrin. AHR activation can modulate inflammatory responses in the skin [19]. It has been shown to regulate the production of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6, as well as chemokines like CXCL8/IL-8. AHR signalling influences the function of immune cells in the skin, this pathway can regulate the maturation and antigen-presenting capacity of Langerhans cells, which are important for immune surveillance in the epidermis [20]. AHR activation has also been shown to modulate T-cell differentiation and function. Some studies suggest that AHR activation may confer photoprotective effects on the skin and can induce the expression of enzymes involved in the metabolism of harmful UV radiation-induced compounds, such as cytochrome P450 (CYP1A1) [21–23].
AHR has been implicated in the development of skin cancer. Certain environmental pollutants that activate AHR, such as PAHs and dioxins, have been associated with an increased risk of skin cancer. AHR activation can affect cell proliferation, apoptosis, and the expression of genes involved in carcinogenesis [23] (Figure 2).
The role of AHR signalling pathway is relatively well studied in psoriasis [24]. In this condition there is evidence of enhanced AHR activation, primarily driven by endogenous ligands derived from the skin, such as tryptophan metabolites [25]. AHR activation leads to the production of proinflammatory cytokines and chemokines, which contribute to the characteristic inflammatory response in psoriatic skin lesions. Additionally, AHR signalling can affect the balance of immune cells in the skin, promoting the accumulation of pathogenic T cells and inhibiting the activity of regulatory T cells, further contributing to the disease [26]. AHR signalling has also been implicated in atopic dermatitis, a common allergic skin condition characterized by intense itching and eczematous lesions. In atopic dermatitis, AHR activation can lead to increased production of inflammatory mediators and disruption of the skin barrier function, contributing to disease exacerbation [27]. Furthermore, exposure to environmental pollutants that activate the AHR pathway, such as PAHs, has been associated with an increased risk of developing atopic dermatitis [28].
Besides psoriasis and atopic dermatitis, AHR signalling has been investigated in other dermatoses as well. For example, AHR activation has been linked to acne vulgaris, a common skin disorder characterized by sebum overproduction, inflammation, and bacterial colonization. AHR signalling can modulate sebum production and regulate the immune response in the pilosebaceous unit, thereby influencing the development of acne lesions [29]. Overall, AHR signalling plays a complex role in dermatoses, contributing to inflammation, immune dysregulation, and tissue damage. Further research is needed to fully understand the mechanisms underlying AHR-mediated pathogenesis in various dermatoses. Targeting the AHR pathway may hold promise as a therapeutic approach for the treatment of these skin disorders.
Psoriasis
The AHRis observed in cutaneous plaques inside psoriatic skin, where it plays a role in governing both the inflammatory response and the terminal differentiation of keratinocytes and T lymphocytes [10, 26].
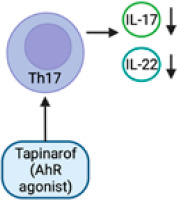
The findings of both in vitro and in vivo investigations have demonstrated that a deficiency in the AHR leads to heightened sensitivity to pro-inflammatory stimuli and the onset of unregulated inflammation [30]. In an imiquimod-induced psoriasis model, AHR-deficient mice displayed substantial psoriasiform skin inflammation and increased IL-17 and IL-22 production as compared to controls. Furthermore, the defective mouse skin models exhibited dysregulation of both keratinocytes and fibroblasts. However, as demonstrated by the fact that inflammation was not reduced when IL-17 was blocked, IL-17 is not the only cytokine implicated in the development of psoriasiform inflammation in the AHR-deficient mouse model [31].The dysregulation of the AHR signalling pathway has been observed in individuals with psoriasis. Numerous studies have provided evidence indicating that the expression and activity of the AHR and its downstream targets undergo modifications within psoriatic skin lesions [32]. The activation of the AHR has been specifically linked to the generation of pro-inflammatory cytokines, including IL-17 and TNF-α [33]. These cytokines have been identified as crucial factors in the pathogenesis and advancement of psoriasis [34]. In recent times, a novel topical therapy known as tapinarof (GSK2894512) has been created and has received approval from the Food and Drug Administration (FDA) for its application in the topical management of plaque psoriasis among adult individuals [35–37].
Tapinarof is a pioneering small molecule topical therapeutic agent that belongs to a new family of compounds. It acts as an AHR-modulating agent, exhibiting the unique ability to bind to and activate AHR [38, 39]. Through this mechanism, tapinarof effectively mitigates skin inflammation, restores the integrity of the skin barrier, diminishes oxidative stress, and regulates gene expression in immune cells [40]. The dysregulation of AHR signalling in psoriasis can be ascribed to various contributing variables. To begin with, there is a suggestion that specific endogenous ligands, including tryptophan metabolites and some constituents of the skin microbiota, possess the ability to activate the AHR and hence contribute to the observed inflammatory response in psoriasis. Moreover, it is worth noting that environmental elements, like cigarette smoke and pollution, have the potential to stimulate the AHR and intensify the inflammatory response associated with psoriasis [41].
Tapinarof (Figure 3) induces the activation of the nuclear factor erythroid 2 related factor 2 (NRF-2) pathway, leading to the production of antioxidative enzymes that serve to restrict oxidative damage. The activation of the transcription factor OVO-like 1 (OVOL1) leads to the creation of loricrin (LOR) and filaggrin (FLG), which are crucial proteins involved in the maintenance of skin barrier function [42]. The activation of AHR by tapinarof leads to the regulation of T-helper (Th17) and T regulatory cell differentiation. This regulation results in the downregulation of IL-17, hence lowering inflammation in psoriatic skin [40] (Figure 4). The mechanism of action of tapinarof is also influenced by its impact on the skin microbiota. Tapinarof has demonstrated antibacterial activity, leading to a subsequent reduction in cytokine production and improvement in the associated disease [18]. In addition, genetic investigations have revealed the existence of genetic variants in genes associated with AHR signalling, which could potentially play a role in the pathogenesis of psoriasis. For instance, there exists a correlation between certain single nucleotide polymorphisms (SNPs) found in genes connected to the AHR and an elevated susceptibility to psoriasis [43]. In summary, there is an increasing body of evidence indicating that AHR plays a crucial function, extending beyond its involvement in maintaining skin homeostasis and integrity, as a promising therapeutic target for inflammatory disorders. Both the AHR ligand and the cellular environment play significant roles in modulating AHR-driven signals, exerting either inhibitory or stimulatory effects on inflammatory processes [44]. The therapeutic targeting of the AHR signalling pathway has been identified as a promising approach for the treatment of psoriasis. Preclinical investigations employing antagonists or modulators of the AHR have demonstrated encouraging outcomes in mitigating the intensity of psoriasis-like cutaneous inflammation in animal models. Nevertheless, additional investigation is required in order to comprehensively comprehend the intricate involvement of AHR signalling in psoriasis and to formulate therapeutic strategies that are both efficacious and devoid of adverse effects, specifically targeting this pathway in human subjects.
Targeting the AHR pathway has emerged as a potential therapeutic strategy for psoriasis. Researchers are investigating the development of AHR antagonists, which could inhibit AHR activation and subsequently dampen the pro-inflammatory response [45]. However, it is worth noting that the precise mechanisms by which AHR contributes to psoriasis are still being investigated, and further research is needed to fully understand its role in the disease.
Hidradenitis suppurativa
Hidradenitis suppurativa (HS) is a chronic debilitating inflammatory skin condition characterized by painful nodules, abscesses, and sinus tracts, primarily affecting areas with apocrine sweat glands, such as the axillae, groin, and perineum. The exact cause of HS is not fully understood, but dysregulation of the immune system and inflammation play a significant role [45, 46]. The aetiology of HS is poorly understood [47]. While mutations in γ-secretase genes were detected in a small subset of patients with HS, their link with the pathophysiology of HS has remained elusive [48]. For the large majority of cases, HS is currently believed to involve defects in genes regulating inflammation and immunity [49]. Concomitant with dysregulated immune responses, metagenomic investigations have revealed the presence of a distinct anaerobic microbiota in the skin affected by HS, with its proliferation exhibiting a positive correlation with the severity of lesions [50, 51]. Inflammatory bowel disease and metabolic syndrome are frequent HS comorbidities [52–54]. In both of these conditions, unresolved inflammation was linked to perturbations of the gut microbiota-host metabolic homeostasis [45].
In recent years, there has been growing evidence suggesting the involvement of AHR signalling in the pathogenesis of hidradenitis suppurativa. AHR signalling has been implicated in maintaining the integrity of the skin barrier. Disruption of the skin barrier allows for the penetration of environmental factors and microbial agents, triggering an inflammatory response. AHR activation may influence the expression of genes involved in skin barrier function and contribute to the dysregulation of the barrier in HS. Studies have shown altered expression of AHR in lesional skin of HS patients compared to healthy individuals. Increased expression of AHR has been observed in the epidermis and hair follicles of HS lesions. AHR can be activated by various endogenous and exogenous ligands. In HS, it has been proposed that the accumulation of endogenous ligands, such as tryptophan metabolites, in the affected skin may lead to the activation of AHR [55, 56].
By controlling the development and operation of immune cells, AHR activation can modify the immune response. The activation of the AHR has been associated with the synthesis of pro-inflammatory cytokines, including IL-17 and TNF-α. These cytokines have been recognized as contributors to the formation of lesions in individuals with HS [57]. IL-17 has been noted as a prominent factor connecting HS with other immune-mediated conditions such as Crohn’s disease, ulcerative colitis, multiple sclerosis, and psoriasis. This cytokine has demonstrated significance as a mediator in the development of HS [56, 57]. The involvement of AHR signalling in the preservation of the skin barrier’s integrity has been suggested. The impairment of the skin barrier facilitates the infiltration of environmental elements and microbial pathogens, hence eliciting an inflammatory reaction. The activation of the AHR has the potential to exert an influence on the expression of genes that are involved in the maintenance of skin barrier function. This activation may also play a role in the dysregulation of the skin barrier in individuals with HS.
Guenin-Macé et al. identified contextual abnormalities in the activation of the AHR, which were observed alongside reduced synthesis of AHR agonists generated from bacteria and a lower prevalence of bacteria capable of synthesizing AHR ligands within the resident microbial community. The expression of the AHR and AHR-dependent genes was examined between samples of healthy and sick skin by researchers. The expression of AHR was shown to be similar across healthy individuals and patients with HS. However, the expression of AHR target genes, AHRR, CYP1A1, and CYP1A2, was significantly lower in patients with HS compared to the healthy control group [55].
HS patients have an alteration in tryptophan catabolism induced by a normal bacterial skin flora, causes alteration in the production of AHR agonists [55]. The diminished activation of the AHR in the affected skin is not a result of inherent deficiencies, but rather stems from the reduced local synthesis of AHR agonists. Agonists of the AHR encompass Kyn, a ligand with modest affinity, the physiological implications of which remain uncertain and indole derivatives originating from bacterial degradation of Trp. Guenin-Macé et al. made metabolomic analysis and among bacterial metabolites of Trp detected indole-3-acetic acid (IAA)- recognized AHR agonist [58, 59]. The levels of IAA were found to be considerably lower in patients with HS when compared to a group of healthy individuals. Hence, the compromised activation of the AHR in skin lesions of individuals with HS aligns with a diminished synthesis of the AHR agonist IAA by the cutaneous microbiota. The dysregulation of tryptophan catabolism occurring at the interface between the skin and the microbiota serves as a mechanism that connects the immunological and microbiological characteristics of HS lesions. The presented data provide evidence supporting the concept that the immunological dysregulation observed in HS skin lesions may be attributed to a modification in the AHR pathway [56] (Figure 5). The involvement of AHR signalling in HS has led to the identification of this pathway as a possible therapeutic target. Several research investigations have examined the efficacy of AHR antagonists in inhibiting AHR activation and mitigating inflammation in individuals with HS [60]. Further investigation is required to have a comprehensive understanding of the involvement of AHR in the development of HS and to ascertain the efficacy and safety of therapeutics targeting AHR.
Figure 5
An alteration in tryptophan catabolism induced by a normal bacterial skin flora, causes alteration in the production of aryl hydrocarbon receptor (AHR) agonists in hidradenitis suppurativa (HS) patients. The diminished activation of the AHR in the affected skin is not a result of inherent deficiencies, but rather stems from the reduced local synthesis of AHR agonists. The dysregulation of tryptophan catabolism occurring at the interface between the skin and the microbiota serves as a mechanism that connects the immunological and microbiological characteristics of HS lesions
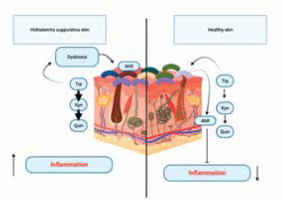
In summary, it may be inferred that the dysregulation of AHR signalling is evident in hidradenitis suppurativa, perhaps playing a role in the persistent inflammation and immunological dysregulation characteristic of this disorder. Additional investigation is necessary in order to elucidate the exact processes by which AHR is implicated in HS and to formulate specific therapeutic strategies.
Acne
Acne is a prevalent dermatological disorder characterized by the obstruction of hair follicles due to the accumulation of sebum, keratinocytes, and microbial agents. The aetiology of acne is intricate and multifaceted, with scientific investigations revealing the involvement of diverse signalling routes and molecular mechanisms, such as the AHR signalling pathway.
The involvement of the AHR has been implicated in the pathogenesis and advancement of acne [61]. A pivotal component involved in the regulation of AHR signalling is a specific molecular entity referred to be an AHR agonist. Research has demonstrated that AHR agonists have the ability to enhance sebum production, an oily material synthesized by the sebaceous glands located within the dermis. The overproduction of sebum can be a contributing factor in the obstruction of hair follicles, resulting in the development of acne lesions.
Furthermore, it has been established that the activation of AHR signalling is associated with the inflammatory response observed in acne, in addition to its role in sebum generation. Activation of the AHR signalling pathway appears to inhibit human sebocytes, reducing sebum production [62]. An in vitro study on the sebocyte showed that AHR stimulation resulted in attenuated expression of lipogenic genes via enhanced SREBP1 proteolysis. The activation of the AHR has the potential to initiate the release of inflammatory mediators, including cytokines and chemokines, that facilitate the process of inflammation and immunological responses within the skin [63]. The inflammatory response has the potential to contribute to the manifestation of redness, swelling, and discomfort commonly observed in acne lesions. The AHR agonist, peptidoglycan, stimulates TLR-2 on cultured human sebocytes to induce TNF-α and IL-8 secretion, effect which was inhibited after AHR knockdown and pre-treatment with an AHR antagonist. AHR ligands such as microbial Trp metabolites may affect lipid synthesis and immune cell differentiation, raising the possibility of therapeutic uses for acne vulgaris treatment [64]. Additionally, the involvement of AHR signalling has been suggested in the regulation of both keratinocyte proliferation and differentiation. The disruption of AHR signalling has the potential to interfere with the regular keratinocyte turnover, hence resulting in the occurrence of comedones and the subsequent onset of acne [65].
A study was undertaken by El Esawy et al. to examine the mRNA levels of AHR, IL-36γ, and IL-38 in a sample of 100 individuals. This sample consisted of 70 patients diagnosed with acne vulgaris and 30 control participants who were deemed to be in good health. The mRNA levels were measured using quantitative real-time PCR.
The study observed a significant increase in the median levels of AHR gene expression among individuals diagnosed with acne vulgaris compared to those in the healthy subjects. The expression levels of the AHR gene exhibited a statistically significant rise in conjunction with greater severity of acne vulgaris [66]. Decreased probiotics in the intestinal tract of acne vulgaris patients reduce microbial Trp metabolites, which are AHR agonists and can alleviate both innate and adaptive immune inflammation, promote the AHR inhibition of sebum production. Supplementation with probiotics may restore Trp metabolism, ameliorating acne vulgaris through an impact on immunity and inflammation. However, direct relationships between probiotics/Trp metabolites and acne vulgaris require further investigation [62, 67]. Ongoing research is being conducted to investigate the precise processes underlying AHR signalling in the context of acne. Further investigations are required to comprehensively elucidate the extent of its involvement and significance in this dermatological condition. Nevertheless, the focus on the AHR pathway has arisen as a prospective therapeutic strategy for the treatment of acne. The regulation of sebum production, inflammation reduction, and restoration of normal keratinocyte function can potentially be achieved by modulating the activation of the AHR using particular agonists or antagonists. This approach offers a promising new direction for the treatment of acne.
It is noteworthy to acknowledge that the involvement of AHR signalling in acne development is merely a single component within the broader context. Acne is a multifaceted disorder that is influenced by a range of factors, such as hormone imbalances, genetic predisposition, and bacterial colonization. The treatment of acne often encompasses a complete strategy that incorporates a blend of topical therapies, oral pharmaceuticals, and personalized adjustments to one’s lifestyle.
Atopic dermatitis
Atopic dermatitis (AD) is a chronic and recurring inflammatory skin condition that is characterized by malfunction in the epidermal barrier and dysregulation of the immune system. The most prevalent risk factor for the development of AD is the presence of loss-of-function mutations in the FLG gene, which plays a crucial role in the creation of the epidermal barrier [68]. Nevertheless, it is worth noting that less than 40% of persons who possess FLG mutations actually exhibit symptoms of atopic dermatitis [68–70]. These observations suggest that other variables are implicated in the development of AD.
Multiple lines of epidemiological research have demonstrated that the exposure of the skin to air pollution might serve as a risk factor for the onset or exacerbation of AD. The primary sources of outdoor air pollutants are predominantly derived from the combustion of fossil fuels in transportation and industrial sectors [28]. These pollutants primarily consist of particulate matter (PM), which encompasses a combination of solid or liquid particles that remain suspended in the atmosphere [71]. Elevated levels of PM in outdoor environments have been found to be correlated with heightened symptoms of AD and the occurrence of itching [72, 73]. A comprehensive meta-analysis consisting of 13 research revealed statistically significant positive associations between exposure to PM and the occurrence of AD [74]. In addition, PM promotes CYP1A1 mRNA expression in human primary keratinocytes in an AHR-dependent manner because it includes PAHs, a prototypical AHR ligand [75]. Furthermore, it has been observed that there is a heightened occurrence of AD in both children and adults who are exposed to tobacco smoke in their homes, in addition to the detrimental effects of PM and active smoking [76]. Tobacco smoke is well recognized as the primary contributor of indoor pollutants, encompassing a multitude of chemical elements, such as PAHs [76]. These studies lend credence to the idea that AHR activation plays a role in AD brought on by air pollution.
There have been reports indicating that the activation of AHR leads to an enhancement in the function of the skin barrier through the acceleration of epidermal terminal differentiation. The precise mechanisms behind the acceleration of keratinocyte differentiation by AHR signalling remain incompletely elucidated. Kennedy et al. [77] highlight the crucial involvement of reactive oxygen species (ROS) formation in this regulatory process. The authors have provided evidence to support the notion that the signalling pathway involving the AHR leads to an increase in the expression of the transcription factor known as OVO-like 1 (OVOL1), and then triggers its translocation from the cytoplasm to the nucleus [6, 10, 27, 37, 78].
The regulation of both filaggrin and loricrin is governed by the AHR-OVOL1 pathway, although the overexpression of involucrin through AHR is not influenced by OVOL1 [79]. Coal tar has been employed as a medicinal agent for the treatment of inflammatory skin conditions, such as AD. Indeed, the administration of coal tar topically has been shown to reinstate the expression of skin barrier proteins, such as FLG, in individuals with atopic dermatitis [80]. Coal tar is known to contain a significant amount of PAHs and has been demonstrated to stimulate the process of epidermal differentiation through the activation of the AHR. The effectiveness of coal tar appears to be linked to the activation of the AHR [80].
Furthermore, tapinarof, which is also known as 3,5-dihydroxy-4-isopropyl-trans-stilbene, is a tiny molecule derived from natural sources. It has demonstrated effectiveness in individuals diagnosed with AD and is currently undergoing clinical trials for the purpose of treating various skin conditions [81]. The patients who received tapinarof cream showed improvement in symptoms associated with Alzheimer’s disease, as observed in a double-blind research conducted during phase 2 [82, 83]. According to a recent study, it was shown that tapinarof exhibits the ability to activate the AHR by directly binding to it as an agonist. Additionally, tapinarof was found to promote the expression of mRNA for epidermal terminal differentiation markers, such as FLG and IVL, in primary human keratinocytes [37]. The utilization of imiquimod has been employed to create skin inflammation that emulates the characteristics of psoriasis. Additionally, it has been observed that tapinarof effectively mitigates the inflammation induced by imiquimod in mouse skin, and this effect is dependent on the AHR [37]. The effectiveness of tapinarof, like coal tar, is believed to be influenced, to some extent, by the activity of the AHR. These comprehensive studies illustrate that the activation of the AHR has a role in repairing the function of the skin barrier. However, it is important to note that an excessive expression of AHR can contribute to the development of AD. The aforementioned research has provided evidence supporting the notion that the AHR holds promise as a therapeutic target. Furthermore, it has been suggested that inducing a moderate degree of AHR activation could potentially be effective in promoting the healing of the epidermal barrier.
Vitiligo
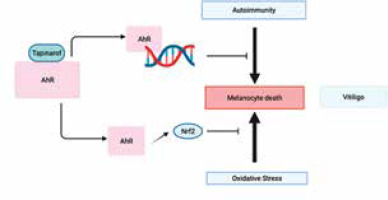
Vitiligo is an autoimmune condition characterized by the death of melanocytes due to immunological cytotoxicity or cell death caused by oxidative stress [84]. Immunosuppressive medications, such as topical tacrolimus, topical ruxolitinib, and narrow-band UV therapy, can be used to achieve repigmentation. Nevertheless, the process of repigmentation frequently requires a significant amount of time, and only a small proportion of patients attain a tolerable level of repigmentation. Hence, it is important to broaden the range of therapeutic alternatives for this condition.
Prior research has demonstrated the involvement of the cytokines IL-17A and IL-22 in the progression of vitiligo [85–88]. The AHR plays a role in the development of vitiligo and has the ability to regulate the production of cytokines. In vitiligo, Liu et al. conducted a study to investigate the connection between AHR activation and CD4+ T cell secretion of IL-17A and IL-22 [89]. A cohort of 20 individuals recently diagnosed with progressing, unstable vitiligo and 20 individuals without any health conditions were selected for the study. CD4+ T cells and skin samples were collected. The following studies were conducted: immunohistochemistry, ELISA, reverse transcription-quantitative PCR, western blotting, and RNA interference. The AHR expression was markedly reduced in the CD4+ T cells and skin, including both lesional and non-lesional areas, of vitiligo patients in comparison to healthy individuals. The expression of AHR was significantly reduced in non-lesional skin of patients with vitiligo compared to their lesional skin. Patients with vitiligo exhibited markedly elevated expression levels of IL-17A and IL-22 in comparison to healthy individuals. Suppression of AHR resulted in a substantial rise in the production of IL-17A and a considerable decrease in IL-22 levels in the CD4+ T cells of vitiligo patients. To summarize, a decrease in AHR expression is linked to the advancement and instability of vitiligo.
Tapinarof (WBI-1001) is a topical nonsteroidal immune modulating agent, first developed by Welichem (Welichem Biotech Inc.). It has been demonstrated to be effective in humans for treatment of atopic dermatitis and psoriasis. It has recently received regulatory approval as a topical psoriasis therapy in China (2019) and the United States (2022) [35–37]. It is an agonist of the aryl hydrocarbon receptor, which is involved in suppressing immune response and inhibiting oxidative stress. Hence, tapinarof has the potential to exert positive effects on vitiligo by inhibiting the two processes responsible for melanocyte demise in the condition [84].
A case report by Liu et al. [90] detailed the three-year history of a white patch on the left cheek of a 19-year-old female student. Off-label use of tapinarof 1% cream was attempted for the vitiligo patches due to the patient’s poor tolerance of topical tacrolimus 0.1% ointment. She used 1% tapinarof cream twice a day without suffering any negative side effects. She noticed repigmentation at the margin after a month, and after 6 month of treatment, she achieved more than 50% repigmentation.
Tapinarof has been shown to inhibit immune-activating cytokines and reduce oxidative stress by activating the production of Nrf2 [37]. Considering the postulated involvement of these two routes in melanocyte death in vitiligo, it is not surprising that tapinarof could potentially treat individuals with vitiligo (Figure 6). Given that topical tapinarof has demonstrated the capacity to cause long-term remission of psoriasis [37] and possesses the capability to diminish the production of memory T cells [91], which play a role in the development of vitiligo [92], it is plausible that it might likewise prompt long-term remission in vitiligo. To properly assess its clinical utility for vitiligo, a randomized double-blind clinical investigation is required.
Summary
The AHR plays a multifaceted role in dermatology. Its involvement in skin cell differentiation, immune responses, sebum production, and response to environmental factors underscores its significance in various dermatological diseases. Targeting AHR signalling pathways may hold promise for the development of novel therapeutic approaches in dermatology.