Introduction
According to global cancer statistics, nasopharyngeal cancer (NPC) is a rare disease, but it poses a great health problem in some regions, including Southeast Asia, Africa, and the Arctic [1]. In Morocco, the annual incidence of NPC is estimated at 1.8%; the majority of patients are diagnosed with locally advanced disease or distant metastasis (80%), and more than 90% of metastatic cases die [2].
Worldwide, the deep anatomic localization of NPC and its late diagnosis limit therapeutic approaches, and radiotherapy is still the main and the most effective treatment of NPC [3]. Scientific evidence has shown that radiotherapy destroys tumour cells by acting directly on the DNA (direct effect), and indirectly by free radicals generated by ionization leading to DNA base lesion (indirect effect) [4]. This DNA injury eventually impacts cell proliferation and changes the cell cycle, inducing cellular apoptosis or activating death pathways [5].
The most important challenge is to overcome radioresistance, considered as the main cause of local recurrence and distant metastasis [6]. In the last decade, research into the molecular basis of radiation response in tumours has improved our understanding of radioresistance, and data clearly shows that radioresistance is mainly due to genetic variations involved in DNA repair pathways. As response to radiotherapy, tumour cells activate a series of complex reactions to survive [7].
Overall, four principal DNA repair pathways occur in cancer cells: mismatch repair, double strand base (DSB) repair, nucleotide excision repair, and base excision repair (BER), being the central DNA repair mechanism to maintain the genome integrity [8]. In this system, two genes, X-ray repair cross complementing 1 (XRCC1) and 3 (XRCC3), play a key role in DNA repair and are considered as important factors in damage signalling and recovery [9].
XRCC1 gene located on 19q13.2, comprising 17 exons and 16 introns, encodes a DNA repair protein that plays a key role in BER pathway by repairing DNA single-strand breaks [10–12]. XRCC1 was the first gene reported to impact cell sensitivity to ionization radiation [13]. The XRCC3 gene located on 14q32.3, covering 10 exons and 9 introns, encodes for the enzyme that participates in DNA DSB repair [10, 11]. Several studies have reported an association between genetic modification of XRCC1 and XRCC3 and the response to radiotherapy in various cancers, including rectal cancer, cervical cancer, breast cancer, and lung cancer [14–19]. In NPC, growing attention has been paid to the association between single nucleotide polymorphisms (SNPs) in DNA repair genes and the response to radiotherapy. In this field, many studies have reported that SNPs in XRCC1 and XRCC3 may act as potential biomarkers for predicting radiosensitivity.
Despite the growing number of studies investigating the predictive role of these SNPs in NPC radiotherapy, the results are still controversial. Recently, Gong et al. highlighted the need of more genetic association studies related to radiation-based treatment to support the interest of considering XRCC SNPs as prognostic factors for patients receiving radiotherapy-related treatment [20]. Nonetheless, we performed this study to evaluate the association between XRCC1 Arg399Gln and XRCC3 Thr241Met SNPs in Moroccan patients with NPC and responses to radiotherapy, to assess their usefulness as prognostic biomarkers of NPC radiotherapy.
Material and methods
Study population and sampling
A total of 100 NPC patient were recruited at Mohammed VI Center for Cancer Therapy in Casablanca between January 2016 and December 2018. Peripheral blood specimens were collected in tubes containing ethylenediaminetetraacetic acid. In this study, all recruited patients were initially scheduled to be treated with radiotherapy. All patients agreed to complete a questionnaire to provide information regarding, age, sex, smoking status, childhood habits, consanguinity, and personal and familial cancer history. Information regarding clinical and pathological status was recovered from their respective medical records. The study protocol was approved by the Ethics Committee of Ibn Rochd University Hospital, Casablanca, Morocco, and each recruited patient signed a written informed consent form.
DNA extraction
Genomic DNA was extracted from leukocyte nuclei using a phenol chloroform DNA extraction method [21]. The concentration of the extracted DNA was quantified using a UV spectrophotometer (Nanodrop, Thermofisher, USA). Then the quality of DNA was checked by polymerase chain reaction (PCR) amplification of a fragment of the β-globin gene using the primers PC04 (5’-CAACTTCATCCA CGT TCACC-3’) and GH20 (5’-GAAGAGCCAAGGACAGGTAC-3’), generating an amplification product of 265 bp.
Single nucleotide polymorphism genotyping
The genotyping of the 2 polymorphic sites in DNA repair genes (XRCC1 Arg399Gln [rs25487] and XRCC3 Thr241Met [rs861539]) was as-sessed by conventional PCR using loci-specific pri-mers, as reported in Table 1.
Polymerase chain reaction amplification was performed in a total volume of 25 µl containing 200 pM of each dNTP, 200 pM of each primer, 0.5 U Platinum Taq DNA polymerase (Invitrogen), and 100 ng of genomic DNA in 1X PCR buffer. Each PCR reaction consisted of DNA denaturation at 95°C for 2 minutes, followed by 40 cycles of denaturation at 94°C for 30 seconds, and primer annealing for 30 seconds at specific temperature and DNA extension for 30 seconds at 72°C. A final extension at 72°C for 7 min was performed for all amplification mixtures. Polymerase chain reaction products were size separated by 1.5% agarose gel electrophoresis and visualized under ultraviolet light.
Table 1
Primer sequences used for of XRCC1 and XRCC3 single nucleotide polymorphism genotyping
| SNP | Primer sequence | Tm (°C) | Amplicon size (Bp) | |
|---|---|---|---|---|
| XRCC1 Arg399Gln | F | 5'-GATCACACCTAACTGGCATCTTC-3' | 54 | 265 |
| R | 5'-CTGGGACCACCTGTGTTC-3' | |||
| XRCC3 Thr241Met | F | 5'-GGTCGAGTGACAGTCCAAAC-3' | 58 | 449 |
| R | 5'-AGCAACGGCTGAGGGTCTT-3' | |||
For SNP genotyping, PCR products were purified using an ExoSAP-IT® kit (Applied Biosystems, USA) to eliminate the primers and the remaining unincorporated dNTPs, and then were subjected to DNA sequencing reaction. Sequencing reactions were performed in a final volume of 10 µl, containing 200 ng of purified PCR product, 1 µl of Big Dyev3.1 ready reaction mix (Applied Biosystems, Foster city, CA, USA), and 10 pmol of the specific primer (the same used for PCR amplification). The sequencing mixtures were incubated at 95°C for 1 min and 25 cycles were performed as follows: denaturation at 95°C for 10 s, primer annealing at 50°C for 5 s, and extension at 60°C for 4 min. Sequencing products were finally purified using Sephadex G-50 gel-exclusion chromatography (GE Healthcare Life Sciences) to eliminate the excess of unincorporated labelled dNTPs. Direct sequencing of amplified DNA was performed by capillary electrophoresis on an ABI 3130XL DNA analyser (Applied Biosystems, Foster city, CA, USA). The sequencing products were analysed using a Basic Local Alignment Search Tool (BLAST) and BioEdit Sequence Alignment Editor to make this analysis complete.
Statistical analysis
The correlation between XRCC1 Arg399Gln and XRCC3 Thr241Met SNPs and clinical stages/response to radiotherapy were evaluated by the χ2 test using SPSS software version 23 (p-values < 0.05 were considered as statistically significant). Survival analyses were performed using the Kaplan-Meier method and compared by a log-rank test using Graph Pad Prism software version 9 (p-values < 0.05 were considered as statistically significant).
Results
Demographic and clinicopathological characteristics
Demographic and clinicopathological characteristics of the 100 recruited patients are summarized in Table 2. In this cohort, males comprised 63% and females comprised 37%, with a male:female sex ratio of 1.70. Overall, 82% of cases were over 30 years old, and 56% had a rural childhood habitat. Most patients had no consanguinity (92%), no familial cancer history (75%), were not cigarette smokers (69%), and were not alcohol consumers (92%). Clinicopathological characteristics of tumour specimens showed that most cases were diagnosed at advanced stages III and IV (86%). Non-keratinising undifferentiated tumour was the most prevalent histological type and was reported in 93% of cases.
Table 2
Demographic and pathological characteristics of the nasopharyngeal carcinoma population study
The distribution of the different XRCC1/3 single nucleotide polymorphism genotypes
The distribution of the different XRCC1/3 SNPs genotypes and alleles in NPC cases is summarized in Table 3. For XRCC1 Arg399Gln SNP, the heterozygous genotype GA, was reported in 27% of cases, whereas the homozygous genotypes GG and AA were identified in 60% and 13%, respectively. Accordingly, the G allele prevails and was reported in 73.5% of cases. The A allele was reported in only 26.5% of cases.
Table 3
Distribution of the XRCC1 and XRCC3 single nucleotide polymorphism genotypes and alleles in nasopharyngeal carcinoma patients
| Genotypes | XRCC1 Arg399Gln | Genotypes | XRCC3 Thr241Met | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| G/G | 60 | 60 | C/C | 36 | 36 |
| G/A | 27 | 27 | C/T | 51 | 51 |
| A/A | 13 | 13 | T/T | 13 | 13 |
| Alleles | Alleles | ||||
| G | 147 | 73.5 | C | 123 | 61.5 |
| A | 53 | 26.5 | T | 77 | 38.5 |
For XRCC3 Thr241Met SNP, the heterozygous genotype CT prevailed and was reported in 51% of cases. The homozygous genotypes CC and TT were reported in 36% and 13% of cases, respectively. Allelic distribution showed a predominance of C allele, reported in 61.5% of cases, T allele being identified in only 38.5% of cases.
Correlation between XRCC1 Arg399Gln and XRCC3 Thr241Met single nucleotide polymorphisms and clinical stages
In this study, correlation between XRCC1 Arg399Gln and XRCC3 Thr241Met SNPs and clinical stages was also evaluated, and the results are reported in Table 4. Genotypic and allelic distributions of XRCC1 Arg399Gln SNP showed no significant association with clinical stages, with p = 0.559 and p = 0.440, respectively. Likewise, NPC clinical stages were not significantly associated with XRCC3 Thr241Met SNP genotypes (p = 0.638) or with corresponding alleles (p = 0.567).
Table 4
Association between XRCC1 and XRCC3 polymorphisms and clinical stages
Association between XRCC1 Arg399Gln and XRCC3 Thr241Met single nucleotide polymorphisms and response to radiotherapy
Among the 100 recruited NPC cases, only 68 underwent radiotherapy, and clinical follow-up revealed that 59 were sensitive to ionizing radiation and showed a positive response to radiotherapy (86.8%), whereas 9 patients exhibited radio-resistance status (13.2%) associated with relapsed, local, or distant disease cases. In this study we also investigated the association between XRCC1 Arg399Gln and XRCC3 Thr241Met SNPs and response to radiotherapy. For both SNPs, statistical analysis showed no significant association between genotypic and allelic distributions and response to radiotherapy (p > 0.05) (Table 5).
Table 5
Association between XRCC1 and XRCC3 polymorphisms and response to radiotherapy
The evaluation of Arg299Gln and Thr241Met single nucleotide polymorphisms as prognosis survival biomarkers in nasopharyngeal carcinoma
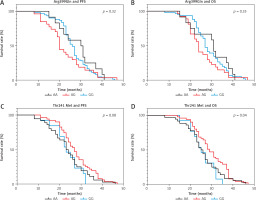
To evaluate whether the Arg299Gln and Thr241Met SNPs has an impact on patients’ clinical outcomes (progression- free survival – PFS, and overall survival – OS) Kaplan- Meier analyses were performed, and the results are reported in Figure 1. Interestingly, patients with CT genotype of Thr241Met SNPs had significantly longer OS (p = 0.04 < 0.05) than those with CC and TT genotypes. However, no statically significant association was detected between Thr241Met genotypes and PFS, or between Arg399Gln genotypes and PFS and OS (p-values > 0.05) (Fig. 1).
Discussion
Radiation therapy is the mainstay primary therapeutic treatment for patients with nasopharyngeal carcinoma. However, despite relevant scientific and technological advances, radio-resistance remains the main problem faced by clinicians in NPC management and the major cause of local recurrence and distant metastasis [22]. The sensitivity of cancer cells to radiotherapy can be strongly influenced by genetic modifications affecting specific genes, including XRCC1 and XRCC3, which are involved in DNA repair processes [7].
In the present study, our interest was focused on evaluation of the association between NPC radioresistance and XRCC1 Arg399Gln (rs25487) and XRCC3 Thr241Met (rs861539) polymorphisms, reported as important factors in the radiotherapy outcomes in several cancers [23].
In this study, no significant relationship was obtained between XRCC1 Arg399Gln polymorphism and NPC patients’ clinicopathological characteristics. Arg399Gln was reported as the most common and important variation of the XRCC1 gene. However, its association with cancer development is controversial. XRCC1 Arg399Gln was reported to be associated with the incidence risk of cervical cancer [5], with lung cancer risk, and with breast cancer risk in African Americans [24]. likewise, in an updated meta-analysis, it was shown that the XRCC1 Arg399Gln polymorphism is a potential predictor for susceptibility to NPC, especially for Asians [25].
In the current study, XRCC3 Thr241Met polymorphism did not reveal any association with clinical stage of NPC cases. Narter et al. previously showed that XRCC3 TT genotype and T allele exerted protective effects against bladder cancer development in a Greek population [26]. In other cancers the results are controversial. Krupa et al. reported no significant association between XRCC3 Thr241Met polymorphism and clinical parameters of colorectal cancer cases [27]. However, a strong relationship between XRCC3 Thr241Met polymorphism and oral squamous cell carcinoma (OSCC) stages has been reported in the Brazilian population, suggesting that this SNP may contribute to the development of OSCC, metastases, and more advanced stages in these lesions [28].
During the last decades, few studies have been conducted to investigate the association between XRCC1/3 polymorphisms and NPC radio-resistance. For instance, no significant association was observed between the XRCC1 Arg399Gln and XRCC3 Thr241Met polymorphisms and the response to radiotherapy (p > 0.05) in our study. In 2006, Andreassen et al. reported no significant association between XRCC1 Arg399Gln and XRCC3 Thr241Met polymorphisms and risk of radiation-induced subcutaneous fibrosis [29].
Evaluation of the association between XRCC1 Arg399Gln polymorphism and radiotherapy outcomes in 60 Chinese patients with locally advanced NPC treated with radiation therapy showed that XRCC1 399 Gln/Gln allele correlated with a higher medium-term tumour regression ratio after radiotherapy for primary nasopharyngeal neoplasm and metastatic lymph nodes (> 80% vs. 40–60%, p < 0.01), suggesting that this SNP is good for prognosis and patients with XRCC1 Codon399 Gln/Gln allele were more likely to obtain complete remission of tumour (100% vs. 76% and 67%, p > 0.05) [30].
The association between XRCC1 Arg399Gln polymorphism and radio-therapeutic response was confronted by several studies in breast cancer [31], head and neck squamous cell carcinoma [32], prostate cancer [33], and non-small cell lung cancer [34].
Interestingly, many studies have addressed the association between XRCC1 399 SNP and radiation side effects. In this field, Chen et al. found that NPC patients with XRCC1 codon 399 Arg/Arg have a higher risk of developing acute radiation dermatitis. Indeed, XRCC1 Arg399Gln polymorphism in NPC suggests that this SNP is an important predictive factor in the risk of acute radiation dermatitis during IMRT [35]. XRCC1 399 SNP was also reported, in combination with smoking, to predict poor PFS in 75 consecutive NPC patients treated with curative radiotherapy [36].
The presence of significant association between XRCC3 241 SNP and radiotherapy outcomes corroborates with previous results on radiotherapy-treated lung cancer patients [37]. Interestingly, Burri et al. reported that XRCC3 had a significant association with the development of late radiation injury in 135 consecutive patients with clinically localized prostate cancer treated with radiotherapy [38].
The association between XRCC3 241 SNP and chemotherapy was also investigated. Shen et al. reported no significant association between XRCC3 Thr241Met polymorphism and clinical outcomes of NSCLC patients treated with platinum-based chemotherapy [39]. However, in a meta-analysis including 14 eligible studies, the predictive value of XRCC3 Thr241Met polymorphism on response and OS of patients with NSCLC was evaluated on 2828 patients, and a significant association was found between the variant XRCC3 241Met and response to platinum-based chemotherapy, while there was no statically significant association between this polymorphism and OS [40].
The XRCC3 Thr241Met variant is a non-conservative substitution and does not affect protein-protein interactions [41]. However, the XRCC1 Arg399Gln mutation leads to conformational changes in the XRCC1 protein, which reduces its affinity for the multi-component DNA repair protein complex [42]. Hence, these mutations may have an impact on protein activity and consequently may be associated with radio resistance status in NPC. Nasopharyngeal carcinoma radio resistance could also be the result of other mechanisms including the presence of cancer stem cells, genetic mutations, some epigenetics events, abnormal activation of certain signalling pathways, and/or alteration of the tumour microenvironment.
Conclusions
XRCC1 Arg399Gln (rs25487) polymorphism is a frequent event in NPC patients, but it is not associated with cancer severity and is not involved in tumour radio resistance. The study suggests that XRCC3 Thr241Met (rs861539) can be associated with different OS in NPC patients treated with radiotherapy. Additionally, other genetic and epigenetic alterations must be investigated to explain the mechanisms associated with NPC resistance for better management of this complicated disease.