Introduction
Internationally, the second most prevalent genitourinary malignant tumour is bladder cancer (BC) [1]. Bladder cancer is one of Egypt’s most prevalent malignancies, representing about 8.7% of cancers in both sexes [2]. For Egyptian men, BC accounts for over 30% of all cancers, which makes it the second most frequent form of the disease [3].
Until recently, surgery consisting of radical cystectomy (RC) with urine redirection, and pelvic lymphadenectomy was the typical procedure for individuals with muscle-invasive bladder carcinoma (MIBC). This procedure shows a 5-year pelvic control rate of 80-90%, and shows a 5-year overall survival (OS) of 40-60% [4]. About 50% of patients experience metastatic tumours despite extensive local treatments, which finally leads to their death [5]. The complications and effects on the quality of life (QOL) are also important. Furthermore, the QOL can be drastically reduced after an ileal conduit, which can change the patient’s physical style and can lead to genitourinary or sexual dysfunction for those who survived after radical cystectomy [6].
According to data accumulated over the past 2 decades, concomitant chemoradiation therapy (CCRT) in MIBC appears to produce results that are comparable to those of RC; nevertheless, we do not have randomized controlled trials to compare between them until now [7].
The use of chemotherapy plus radiotherapy (RT) is justified because the latter alone results in sclerosis at the level of vasculature over months; on the other hand, chemotherapy alone will not be effective [8]. However, some chemotherapeutic agents (e.g. cisplatin and gemcitabine) possess radio-sensitizing properties, which supports the combination of both therapies [9]. Choudhury et al. showed that patients receiving concomitant chemoradiotherapy with gemcitabine in a phase II trial had increased ratios of response, long-term localized control, and tolerable toxicities [10].
It was found that bladder function, which significantly affects QOL, did not become substantially worse with the use of chemoradiotherapy. The use of chemotherapy and radiation therapy in BC survivors with many comorbid conditions showed substantial advantages, with accepted long-lasting bladder activity and decreased salvage cystectomy ratio.
The preservation of the urinary bladder as a therapeutic option for MIBC has been found to be effective in international guidelines for BC. The guidelines of the National Comprehensive Cancer Network reported that preservation of the urinary bladder with chemotherapy and radiation, after maximal transurethral resection of bladder tumours (TURBT), is an alternative treatment option to RC for MIBC [11].
The elimination of malignancy and micro-metastases along with ideal QOL are crucial for MIBC; this continues to be a complex challenge. Triple-mode therapy with extensive TURBT, radiation, and chemotherapy, with results comparable to surgery, has emerged as a promising bladder-preservation technique due to improved QOL and retaining the patient’s ability to control bladder function [12].
Neoadjuvant chemotherapy (NACT) has been added to CCRT because there is evidence that micro-metastases coexist with invasive BC. A study reported that after 2 years, the risk of developing distant metastases because of resection is about 50% [13]. Therefore, it is hypothesized that combined treatment would be effective in reducing the risk of distant metastasis by eradicating micro-metastatic pathologies. Accordingly, the best time to attack possible micro-metastatic cells is before the onset of overt metastatic disease.
So, NACT with locoregional control can fix micro-metastases and improve survival [14]. Two meta-analyses in 2003 and 2005 made by advanced BC provided proof that NACT use increases survival [15, 16].
The aim of this research is to analyse the effectiveness and safety of the bladder preservation approach based on TURBT and then NACT with CCRT in the treatment of patients with MIBC, and to evaluate the patient’s response with calculation of overall survival.
Material and methods
Between January 2018 and March 2021 this prospective research was carried out at the Hospital of Zagazig University, Egypt, by triple-department co-operation with the Medical Oncology, Urology, and Clinical Oncology and Nuclear Medicine Departments. This study included 34 patients diagnosed with de novo MIBC, who were indicated for neoadjuvant chemotherapy but were unwilling to undergo cystectomy because they wanted to preserve their bladder. After being fully informed of the benefits and risks of the bladder-preservation technique vs. RC, patients enrolled in the trial based on their personal preferences.
Inclusion/exclusion criteria
Patients had to meet the following criteria before beginning treatment: muscle-invasive transitional-cell bladder carcinoma with histological confirmation. Participant’s age of 18 years or older. Eastern Cooperative Oncology Group performance status ≤ 1. Tumour meets resection criteria. Tumor, Node, Metastasis (TNM) muscle invasive stage (T2–T4a without nodal involvement or metastasis).
Patients have adequate haematological (haemoglobin ≥ 10 g/dl, WBC ≥ 3000 mm3, neutrophils ≥ 1000/mm3, and platelets equal or above 100,000/mm3), hepatic (alanine aminotransferase, aspartate aminotransferase less than double normal, and bilirubin < 1.5 mg/dl), and renal functions without hydronephrosis (creatinine < 1.5 mg/dl and creatinine clearance ≥ 60 ml/min).
Neither pelvic radiation nor systemic chemotherapy had been administered previously.
Patients with hearing impairment or neuropathy were not included in our study because it can impair the QOL, which should be kept adequate for our patients. Written informed consent was obtained from each patient. Institutional Review Board (IRB) approval was obtained (IRB No. 9235).
Pre-treatment evaluation
Baseline assessment included history and physical examination with full haematological investigations including hepatic function, renal functions, creatinine clearance, and complete blood count. Tumour staging was determined by computed tomography (CT) of the chest, abdomen, and pelvis. All patients underwent cystoscopy examination. Bone scanning and pelvic magnetic resonance imaging (MRI) were done when indicated.
Treatment protocol
After maximal TURBT, 3 cycles of chemotherapy induction followed by concurrent chemoradiotherapy were given to all patients included in the study.
Neoadjuvant chemotherapy
All patients received planned NACT with complete blood cell, liver, and renal functions to be reviewed before each cycle. Cisplatin (70 mg/m2/day on day 1 with appropriate hydration) and gemcitabine (1000 mg/m2/day on days 1 and 8). The combination therapy was administered for 3 cycles repeated every 21 days (3 weeks apart).
When we faced borderline renal functions, we did cisplatin dose splitting on day 1 and day 8 as 35 mg/m2. To evaluate the severity of chemotherapy-related side effects, we used the National Cancer Institute Common Toxicity Criteria, Fourth Edition.
Assessment of response post NACTH
After 4-6 weeks of 3-cycle chemotherapy, all patients were evaluated by cystoscope while radiological investigations (CT or MRI) were preserved to evaluate the response of the tumour by applying Response Evaluation Criteria in Solid Tumours (RECIST). Those who did not achieve benefit (failed to have complete response – CR, or partial response – PR, ≥ 50%) were offered cystectomy; 4 patients agreed and underwent cystectomy.
Concurrent chemoradiotherapy
Concomitant platinum-based chemotherapy with cisplatin (30 mg/m2/week) was given by infusion over one hour beginning from the first day and continuing throughout the RT course. After the chemotherapy infusion was finished, radiation treatment was started.
Computed tomography scans were obtained for all patients undergoing RT with 5-mm slices. The scan was done in the supine position; 3 radiopaque markers were placed using a laser localizer device to ensure positional consistency. These predetermined landmark locations served as the standard point for comparison.
The ELEKTA dual-photon energy with 6 and 10 mV linear accelerator was used to administer the radiation therapy. Our 3-dimensional conformal radiotherapy treatment protocol included 2 phases: whole pelvic irradiation to subclinical disease (pelvic lymph nodes, i.e. peri-vesical, obturator, external iliac, and hypogastric lymph nodes) by 22 fractions; fractions were divided as one fraction per day at a dose of 44 Gy and 5 fractions per week with fixing 4-field box procedure to the entire pelvis. A full bladder was essential for all patients. After that, the bladder as a whole received 20 Gy in 10 parts utilizing the 3-field approach as a boost with a safety buffer. This was delivered using a linear accelerator utilizing a 3-field approach with photon energies of 6 and 10 mV depending on separation and specific individual patient characteristics. In the second phase, the patient was instructed to have an empty bladder.
At least 95% of the planning target volume should be covered by the prescribed doses. The total radiation dose introduced to the bladder after the end of therapy was 64 Gy. The maximal dose of radiation that entered the femoral heads was 45 Gy, and it was 55 Gy for the posterior wall of the rectum. The rectum was subjected to a dose constraint of V55 50%, and both femoral heads and the intestines were to a dose restriction of Dmax 45 Gy.
Follow-up and treatment evaluation
Medical history, examination, urine analysis, radiological evaluation (CT/MRI), and cystoscopy were obtained. After 6 weeks of CCRT, a cystoscope and radiological investigations (CT or MRI) were done to evaluate the response of the tumour by applying RECIST [17]. Responses to therapy were defined as World Health Organization (WHO) criteria (complete, partial, stable disease, and progressive disease – PD) [18]. For the first 2 years, patients were examined every 3 months, then every 6 months.
In the European Organization for Research and Treatment of Cancer (EORTC) and the Radiation Therapy Oncology Group, toxic parameters were used to evaluate late treatment-related toxicity (EORTC) [19].
Statistical analysis
The medical records provided information on the clinicopathological features. Survival, calculated from the date of diagnosis until the date of death, and the OS were determined. From the date of diagnosis to the date of treatment failure, disease-free survival (DFS) was calculated (recurrence of disease or distant metastasis). The data were evaluated using a statistical package software system. For quantitative data, descriptive statistics was represented as values and percentages. The ANOVA and χ2 tests were used to analyse the significance of differences between the variables. The Kaplan-Meier method was used to produce survival curves. The log-rank test was used to assess group survival. For all statistical tests, a p-value of less than 0.05 (2-sided) was regarded as statistically significant.
Results
In our study, 34 patients were enrolled and were evaluated for response. They received 3 cycles of full-dose NACT without dose reduction. At the beginning, the average age was 55.5 years (range: 44–67 years). 82.4% of patients were male and 17.6% were female. Eighteen patients (52.9%) were with radiological stage > T3N0M0. Performance status was 0–1. All patients had transitional cell carcinoma on a histological level. The patients’ data and disease parameters before treatment are described in (Table 1).
Table 1
Patients and disease characteristics
After maximal TURBT was performed, the overall NACT response rate was 79.4% (27 patients) with a CR rate of 61.7% (21 patients). Additionally, 6 patients (17.6%) showed PR > 50%. All obtained radiology, cystoscopy, and numerous random biopsies provided confirmation. Seven patients (20.5%) showed PR < 50%, stable disease (SD), and PD; 4 of them underwent RC followed by postoperative radiation. The average follow-up period was 46 months (range: 6–52 months). Based on the disease status at the most recent follow-up, the pattern of failure was examined. Local recurrences occurred in 7 (24.9%) and distant metastases in 6 (22.2%) of the 27 patients who received CCRT. At the most recent follow-up, 20 patients (58.8%) still had their functional bladders. However, 3 of the 4 patients who underwent RC followed by adjuvant therapy developed metastases at nearby and distant sites.
Out of the 34 patients who had been followed up, 23 patients (67.6%) were still alive. Three-year OS and DFS were, respectively, 70.8% and 65.1%, as shown in Figures 1 and 2. Complete response had a better 3-year DFS (76%) than PR > 50% (40%). Three-year OS rates were 85.7% for those who attained CR, but they fell to 75% for those who had PRs greater than 50% and 21.4% for those who had either PRs less than 50%, SD, or PD (Table 2).
Fig. 1
Kaplan-Meier survival curves illustrating the progression-free survival (months) in classic transurethral resection of the bladder tumour patients vs. no classic transurethral resection of the bladder tumour
cTURBT – classic transurethral resection of the bladder tumour
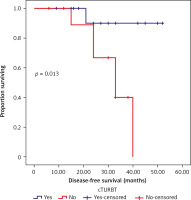
Fig. 2
Kaplan-Meier survival curves illustrating the overall survival (months) in patients with partial response
PR ≤ 50%, stable disease or progressive disease vs. PR > 50%
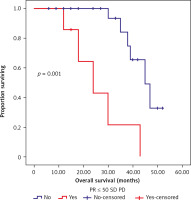
Table 2
Disease-free survival and overall survival analyses with Kaplan-Meier method using log-rank test
Poor responders (with PR 50%, SD, or PD) to NACT had significantly worse DFS and OS outcomes on univariate analysis when compared to those who either had CR or PR > 50% to NACT. Disease-free survival and OS results with cTURBT were significantly superior to those without (Fig. 3, 4, Table 3).
Fig. 3
Kaplan-Meier survival curves illustrating the overall survival (months) in classic transurethral resection of the bladder tumour patients
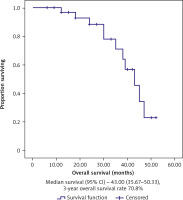
Fig. 4
Kaplan-Meier survival curves illustrating the disease-free survival (months) in classic transurethral resection of the bladder tumour patients
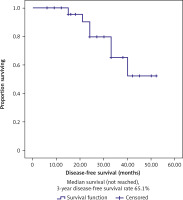
Table 3
Univariate analyses for survival ends (disease-free survival and overall survival)
In terms of DFS and OS, patients under 60 years old and without comorbidities did not significantly differ from their control groups (Table 3). Poor responders (PR 50%, SD, and PD) to NACT and cTURBT demonstrated statistical significance for DFS and OS outcomes on multivariate analyses (Table 4).
Toxicity
The majority of the toxicities associated with treatment were gastrointestinal. Twenty-eight patients experienced grade II nausea, 16 experienced grade II vomiting, and 2 experienced grade III vomiting. Similarly, 20 and 10 patients experienced grade I and grade II diarrhoea, respectively. Grade III, II, and I urinary frequency were noted in 2, 13, and 11 patients, respectively. Nine patients showed grade I anaemia while 6 patients showed grade II as a result of acute haematological toxicity. Blood transfusions were used to treat 2 patients who had developed Grade III anaemia. Seven and 3 patients had grade I and II neutropaenia, respectively. Eight and 5 patients showed acute thrombocytopaenia grade I–II, respectively.
Each patient finished the prescribed radiation therapy regimen. During irradiation, there were no complications that were worse than grade II. In the present study, there were no serious side effects from NACT and concurrent CCRT. Most of them were grade 1 or 2 with no need for treatment discontinuation or interruption, with good quality of life, better tolerability, and compliance.
Discussion
Bladder cancer is one of the most prevalent urological malignancies. Muscle-invasive bladder carcinoma represents nearly 25–30% of patients diagnosed with BC for the first time [20]. Muscle-invasive bladder carcinoma is treated with RC with urinary diversion and bilateral pelvic lymph node dissection [21]. Males should have the prostate and seminal vesicle removed concurrently, while females should have the uterus, bilateral adnexa, and part of the anterior wall of the vagina removed concurrently [20]. Because there are so many surgical resections, the procedure takes a long time and there are many complications. After the surgery, patients’ QOL is gravely compromised. Also, young patients’ fertility function will be lost, and the majority of patients’ sexual function will be lost; eventually, many patients will choose to abandon the surgery [22].
Maximum transurethral resection, systemic chemotherapy, and RT combined with bladder preservation have produced 5-year survival rates comparable to cystectomy, with 50–60% of patients surviving with their bladders intact. The 2015 release of UK BC treatment guidelines suggested that patients with MIBC can be given the option of RC, or RT with a radiosensitizer [23].
The median age in our research was 53 years. The median age in a study by Zhong et al. was 66 years for preservation [24], and it was 61 years in another study with the same design. Dracham et al. documented that the median age at presentation was 62 years (range 50–74 years) in their study [25]. In some other studies, older patients’ ages were reported for cystectomy (67 years and 75 years) [25]. In only one study, an older median age of patients – with a median of 82 years – was openly chosen for cystectomy [26].
So, compared to study reports, our population mean age is younger, with patients’ preference to select preservation rather than RC. In our research, BCr incidence was reported to be higher in men (82.4%), which agrees with the incidence among males in other Egyptian studies about BC (80%), with a male to female ratio of 4 : 2 [3]. In addition, a systemic review and meta-analysis reported that 80% of bladder preservation cases were males [27]. Interestingly, in another study, males made up to 97% of bladder preservation cases [23].
In the present study, the majority of patients were with radiological stage > T3a (52.9%), 29.4% of patients wereT2, and 17.6% patients were T4a. This corresponds to the stages reported in a study by Zhong et al. in which 53% of participants were T2, nearly one-third (33%) of participants were T3, and 14% of them were T4 [24]. In a study by Dracham et al. T2 comprised 45% of cases, T3 comprised 40%, and T4 represented 15% of cases [25].
In our study, open rectal resection (ORR) to NACT was calculated as 79.4%, while Dracham et al. reported 87.5% ORR to NACT. However, our CR was slightly higher than in the Dracham et al. study (61.7% vs. 22.5%, respectively); this is most likely due to cTURBT, which was not determined in their study [25].
Some studies reported slightly lower rates of CR, such as Baxter et al. [28]. Fahmy et al. reported in their meta- analysis that 75.3% of the patients achieved CR [29].
According to Giacalone et al., who reported long-term results of patients with BC treated with tri-modality therapy, CR increased from 66 to 88% between 1986 and 2013 [30]. The research used maximal TURBT, 2 cycles of NACT with Gemzar/Cisplatin, followed by RT with or without radiosensitizer, and revealed an overall response rate of 78%, CR at 30%, PR at 48%, and SD at 22%. Our study yielded comparable results, with an overall NACT response rate of 79.4% [31].
In this research, 52.9% of patients had not achieved cTURBT initially; they had a statistically significant lower PFS compared to those who had cTURBT (p-value = 0.01) and poorer OS (p-value = 0.022). These findings are similar to the results of Zhong et al., which showed that 60% had incomplete TURBT with lower PFS (p-value = 0.029) [24]. In our study, 4 patients (11.7%) underwent salvage cystectomy. The salvage cystectomy rate in the Mitin et al. study was 30%, which is considerably higher than the rate in our study. It might be a result of the patient refusal in our study, as well as the radiation dose being lower in their study, at 46 Gy [32]. Also, salvage cystectomy rates ranged from 0 to 55% in some studies [33, 34].
The local recurrence rate for bladder preservation after 3 years was 29.4% in our study. In bladder preservation, Huddart et al. found a 68.9% local recurrence rate. Although many patients did not receive radiosensitizers, the bladder was the only target volume of the RT given to their patients with the addition of a safety margin [35]. In addition, Kim et al. reported a 74% local recurrence rate at 5 years in patients who underwent preservation of the bladder. However, only 24% of the patients in their study received NACT while 28% of the patients received RT alone [36]. Our result showed a 3-year OS of 70.8%. In a recent study from Canada by Kulkarni et al., a retrospective propensity score-matched analysis in MIBC patients between TMT (n = 56) and RC (n = 56) showed similar survival outcomes (76.6% vs. 73.2%, p = 0.49) [37].
In 2011, a Cochrane database of systematic reviews included 2809 patients in 10 randomized trials, which presented comparative findings. NACT in combination with cisplatin led to a 4% improvement of 5-year OS (45% vs. 49%) and an 11% decrease in the risk of death. This supports the promising role of NACT, which may be the best choice in MIBC after organ preservation. This lends credence to our trial’s promising role of NACT followed by organ preservation in MIBC [24]. Fahmy et al. performed a meta-analysis that included 57 studies on the preservation of bladder and RC treatment protocols for long-term oncological outcomes; they discovered that the 10-year OS showed no statistically significant difference, which was 30.9% vs. 35.1%, respectively (p = 0.32) [29].
Although Zhong et al. and Seisen et al. reported a preliminary RC OS benefit vs. bladder preservation, they ultimately concluded that the possibility of operative mortality may lessen the long-term benefit of RC. The statistical significance of the propensity-matched analysis may be explained by the fact that older patients with comorbid conditions are given the bladder preservation option [24, 38].
Conclusions
Finally, bladder preservation provides considerable survival with high QOL for MIBC patients with tumour grades T2–T4a. The results demonstrate good survival rates and freedom from metastasis. The following are some limitations of our study: first, the sample of patients was small; second is the short follow-up period; more accurate results could be achieved if the follow-up period was prolonged. Further studies with a larger sample size and longer follow-up period are recommended.








