Introduction
Chronic liver diseases are regarded as a significant worldwide health burden causing high morbidity and mortality [1, 2]. Chronic liver damage without propertreatment eventually progresses to advanced liver fibrosis or cirrhosis, which are significant risk factors for hepatocellular carcinoma (HCC), a condition that affects millions of individuals worldwide [2]. Cirrhosis and fibrosis of the liver are chronic inflammatory illnesses where excessive wound healing is associated with several causes, including alcohol addiction, viral infections, metabolic disorders, immune disease and some genetic factors [3-6].
The transforming growth factor (TGF) family of growth factors includes the multifunctional growth factors known as bone morphogenetic proteins (BMPs), which control cell proliferation, apoptosis, and differentiation [7]. They play pleiotropic roles in diverse biological functions in the digestive tract, brain, heart, gonads, skin, and liver [4]. The abnormal expression of bone morphogenetic protein (BMP)-4, BMP-7, and BMP-9 is demonstrated to be associated with HCC development [8]. BMP-7 is an approximately 35 kDa glycoprotein expressed by several tissues, including the reproductive system, muscle and bone, heart, liver, kidney, brain, etc. [9]. It plays a significant role in renal development and stimulates the production of ectopic bone and cartilage [10]. BMP-7 is a vital protein in organogenesis and liver development [11]. It has also been demonstrated to have anti-tumorigenic potential in numerous human cancers, such as breast cancer and glioblastoma [12]. BMP-7 serum levels were elevated in patients with chronic liver disease, and they were significantly higher in patients with liver cirrhosis compared to chronic hepatitis C and HCC cases [13]. Furthermore, BMP-7 has been shown to inhibit liver fibrosis, which is a known major risk factor for developing into HCC [1]. The studies on renal fibrosis have provided most of the information on the function of BMP-7, which shows a protective anti-fibrotic impact by inhibiting TGF-β induced epithelial-mesenchymal transition [2, 14]. Additionally, it was discovered that blocking BMP-7 signaling promoted the invasive development of HCC cells [15]. Better understanding of the single gene mutations in the genes that stimulate tumor cell proliferation, angiogenesis, invasion, and metastasis has resulted in the identification of prospective treatment targets. Hence single nucleotide polymorphisms (SNPs) may affect a person’s susceptibility to particular diseases and infections [16, 17].
This study was designed to explore the relationship between the BMP-7 (rs230205 A/G) SNP and susceptibility to HCC development from liver cirrhosis in the Egyptian population. Moreover, we evaluated the impact of the BMP-7 rs230205 (A/G) SNP on the BMP-7expression level in both cirrhotic and HCC patients and its possible correlation with HCC progress. To the best of our knowledge, this study is the first one investigating the rs230205 [A/G] SNP in cirrhosis and HCC patients.
Material and methods
Subjects
Our study was conducted on 150 participants categorized into 100 patients vs. 50 age- and gender-matched healthy individuals with no previous history of viral infection or any other diseases enrolled as controls. The patient group was divided into 50 cirrhotic post-hepatitis C or B viral infection patients without HCC development (cirrhosis group) and 50 cirrhotic posthepatitis C or B viral infection patients who developed HCC (HCC group). All participants were subjected to complete clinical and laboratory investigations. Patients’ histories were taken including age, sex, full medical history, therapy and any previous HCV therapy or HBV therapy. According to established diagnostic criteria, HCC patients were identified by the characteristic vascular enhancement pattern detected by triphasic computed tomography (CT) scanning. All subjects with immunosuppression, human immunodeficiency virus infection, receiving chemotherapy and antiviral medication or any other form of cancer were excluded. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Menoufia University. Institutional Review Board (IRB) number: 00411/2022.
Genotyping of BMP-7 rs230205 (A/G) SNP
Blood sampling
Peripheral venous blood was collected from all enrolled subjects and withdrawn into 2 sterile tubes. One containing ethylene-diamine-tetra-acetic acid (EDTA) was used for complete blood count (CBC) including total leucocytic count (TLC), hemoglobin and platelet count using an automated cell counter (Sysmix KX-21, Japan) and for deoxyribonucleic acid (DNA) extraction. The other tube was a plain sterile collection tube in which the blood was allowed to clot then centrifuged and serum was separated, aliquoted for biochemical investigations and stored at −80°C for evaluation of the serum levels of BMP-7 protein. Biochemical investigations including the assessment of liver functions, namely, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, total and direct bilirubin and the tumor marker α-fetoprotein (AFP) using EIA (COBAS-Amplicore, Germany), HCV Ab and HBsAg were done by third generation enzyme-linked immunosorbent assay (ELISA).
DNA extraction
Using whole blood samples, the extraction of DNA was done using the salting out extraction method [18]. The integrity of extracted genomic DNA was verified by performing 1.5% agarose gel electrophoresis, while DNA concentration was verified by a Nanodrop 2000c (Thermo Scientific, USA).
Genotyping
The tetra-primer ARMS-PCR method was used to assess the SNP (rs230205) polymorphism in the BMP-7 gene. The selection of the SNP was according to PubMed published data (SNP database). A Mastercycler Gradient thermocycler (Eppendorf, Germany) was used for all amplifications. DNA samples were initially denatured at 94°C for 10 min, followed by 35 cycles of denaturation at 94°C, annealing at 62°C and extension at 72°C for one min. Primers were designed using PRIMER1 online software (http://primer1.soton.ac.uk/primer1.html, 10 January 2021) and the sequences are shown in Table 1. The A allele product appeared at 547 bp, the G allele at 305 bp and the control band at 712 bp. The four primers were used in the simultaneous amplification processes in one reaction tube. Following the separation on 1.5% agarose gels (SigmaAldrich, Germany), the ethidium bromide-stained PCR products were visualized on a UV trans-illuminator.
Assessment of BMP-7 expression levels
The ELISA method was used for the BMP-7 quantitative assessment in serum of studied subjects. The method was carried out using a human BMP-7 ELISA Kit (Sunred Biological Technology, China) according to the manufacturer’s protocol. At 450 nm, the absorbance was read using a microplate reader (Biotech, Inc., USA).
Statistical analysis
SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) was used for data analyses. To determine the normality of the distribution, the Shapiro-Wilk test, one of the normality tests, was carried out. One-way ANOVA and Kruskal-Wallis tests followed by suitable post hoc tests were used to compare the studied groups. The qualitative variables were analyzed by the χ2 test. The Jonckheere-Terpstra test was applied to detect the increasing or decreasing trend among the ordered groups. The Mann-Kendall (M-K) test was applied for detection of the presence of linear or non-linear trends (steadily increasing/decreasing or unchanging) in data series by assessing the effect size following the Jonckheere-Terpstra (J-T) test. Pearson and Spearman correlation with 95% CI were applied to assess the strength and direction between the studied markers. For the estimation of sensitivity, specificity, accuracy, positive and negative predictive values, receiver operating characteristic (ROC) curve analysis was performed. P-values are statistically significant if less than 0.05.
Results
Patients’ features
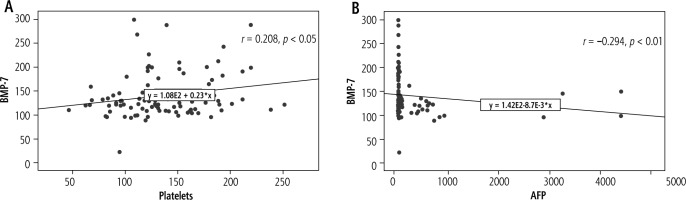
In the current study, the demographic data of all subjects are presented in Table 2. Age and sex of patients and controls showed no significant difference. The hematological and biochemical investigations of all enrolled subjects are described in Table 3. It was found that hemoglobin (Hb), platelets, albumin and TLC revealed a significant decrease across the groups (p < 0.001). HCC patients showed a significant increase in total bilirubin levels (p < 0.05), direct bilirubin (p < 0.01), AST (p < 0.05) and AFP (p < 0.001). A significant decline (p < 0.05) in platelet count was noted in HCC patients compared to cirrhotic ones.
Table 2
Demographic data among the studied participants
Table 3
Laboratory investigations of the studied groups
Genotypes and allelic distribution
The allelic frequency of BMP-7 rs230205 (A/G) was evaluated using ARMS-PCR, and the PCR amplicons were separated using agarose gel (Fig. 1). Genotyping for patients and healthy controls are displayed in Table 4. Our results indicated that the frequency of BMP-7 rs230205 (A/G) genotypes was within Hardy-Weinberg equilibrium in control [25, 11 and 4 (observed) vs. 23.3, 14.5 and 2.3 (predicted), p > 0.05], cirrhosis [30, 14 and 6 (observed) vs. 27.4, 19.2 and 3.4 (expected), p = 0.054] and HCC [28, 15 and 7 (observed) vs. 25.2, 20.6 and 4.2 (expected), p > 0.05] groups.
Fig. 1
Representative digital photograph of ARMS-PCR amplified products separated on 1.5% agarose gel electrophoresis showing the BMP-7 (rs230205) genotyping against Gene Ruler 1 kb DNA ladders (Thermo Scientific, O’Gene Ruler, USA)
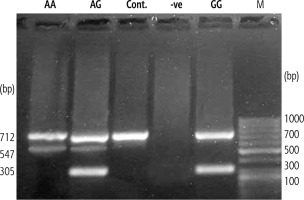
Table 4
Genotype and allele distribution of BMP-7 rs230205[A/G] SNP among different groups
Analysis of the BMP-7 rs230205 (A/G) SNP revealed that the GG genotype and G allele were the most frequent genotype/allele in the three studied groups, whereas the AA genotype and A allele were the least frequent genotype/allele. No significant difference was recorded in the distribution of all genotypes and alleles in rs23020 (A/G) SNP between cirrhotic patients, HCC patients and controls. Thus, there was no significant association between polymorphism in rs230205 (A/G) of the BMP-7 gene and the development of cirrhosis or progression to HCC.
By groups’ segregation, the AA genotype (OR = 1.75, 95% CI: 0.45-6.79) and A allele (OR = 1.50, 95% CI: 0.77-2.93) might be considered a risk factor for cirrhosis. Furthermore, the AG and AA genotypes and A allele could be risk factors for HCC [OR = 1.70 (95% CI: 0.68-4.29), OR = 2.19 (95% CI: 0.58-8.23), OR = 1.74 (95% CI: 0.90-3.37), respectively]. Looking at the OR values, no genotype or allele could be considered a risk factor for the development of cirrhosis to HCC.
Association between BMP-7 SNP and laboratory investigations
The AG genotype carriers showed a significant reduction in albumin level compared to carriers of GG genotype (p < 0.05) (Table 5).
Table 5
Association between laboratory investigations and BMP-7 rs230205 [A/G] genotypes
Plasma levels of BMP-7 in patients and controls
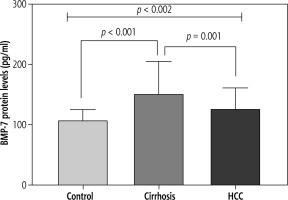
BMP-7 protein showed a significant elevation among the studied groups where a p value for the J-T test of 0.022, 0.19 (0.03-0.34) was found. Post hoc analysis revealed that both cirrhosis (152.9 ±52.2 pg/ml) and HCC (126.3 ±34.2) had a significant level of BMP-7 compared to controls (107.6 ±18.3) (p < 0.001) while cirrhosis was significantly higher than HCC (p < 0.01) (Fig. 2).
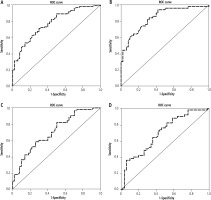
The different area under curve (AUC) values and parameters of validity of BMP-7 protein including sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) are displayed in Table 6. The highest AUC was reported between cirrhosis and controls (AUC = 0.843, sensitivity = 82% and specificity = 70%) (Fig. 3A-D).
Table 6
Validity of BMP-7 between studied groups
Association between BMP-7 SNP and expression level
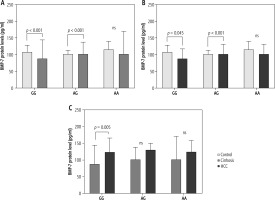
As displayed in Figure 4, compared to controls, cirrhotic patients showed an elevation of BMP-7 levels with all genotypes. GG and AG carriers had a significant rise in BMP-7 level (p < 0.001, p < 0.001) (Fig. 4A).The same results were observed in HCC patients compared to control subjects. The GG and AG carriers had a significantly increased level of BMP-7 protein (p < 0.05, p < 0.001) (Fig. 4B). Thus, the presence of the G allele could be responsible for the elevation of BMP-7 protein.
Regarding the development of the disease, HCC patients recorded a reduction in the protein levels in all genotypes compared to cirrhotic ones. A significant diminution in BMP-7 protein was found in HCC patients carrying the GG genotype compared to cirrhotic ones who were GG carriers (p < 0.01) (Fig. 4C). Consequently, the GG genotype might be responsible for BMP-7 reduction with disease development.
Discussion
Bone morphogenic proteins are multipurpose cytokines that control different cell types’ development, differentiation, and apoptosis [8]. The abnormal expression of BMP-7 is reported to be associated with HCC progress [19]. In this study, we aimed to evaluate the impact of genetic polymorphism of BMP-7 (rs230205) on the expression of BMP protein and its correlation with cirrhosis progress into HCC. Our results indicated that the BMP-7 rs230205 GG genotype and G allele were the most frequent genotype/allele in our groups. The current study data reveal no significant differences in the distribution of all genotypes and alleles in the rs230205 (A/G) SNP between cirrhotic patients, HCC patients and controls. On the other hand, the results indicate that the AA genotype and A allele might be considered as risk factors for cirrhosis. Furthermore, the AG and AA genotypes and A allele could be risk factors for HCC. In contrast, no genotype or allele could be deemed to be a risk factor for the development of cirrhosis to HCC. To our knowledge, only one study has evaluated the impact of this SNP on postmenopausal Chinese women. It suggested that BMP-7 (rs230205) is not associated with osteoporotic fractures or bone mineral density [20]. BMP-7 is a pleiotropic growth factor that is essential for the growth of a variety of tissues and organs [9]. It is considered to be a regulator of endogenous control of liver homeostasis and hepatocyte proliferation [21]. For the BMP7 gene SNP rs162316, El-Shahat et al. found that the A allele (AG genotype) was significantly associated with progression of HCC. However, AG genotype of rs162316 may be considered as a predictor for HCC development in Egyptian cirrhosis patients [21]. BMP-7 levels were detected to be correlated with HCC [13]. Overexpression of BMP-7 reduced liver fibrosis, which may develop into HCC, and the inhibition of its signal facilitated HCC invasiveness [1, 15].
In this study, we observed a significantly high expression level of BMP-7 protein in cirrhosis and HCC patients compared to healthy subjects. Moreover, BMP-7 was significantly higher in the cirrhotic group compared to the HCC group. Li et al. [19] noted a high expression level of BMP-7 in HCC cells compared to normal liver cells and reported it as an oncogene with prognostic significance in HCC, which was speculated to be a potential target of anti-angiogenic therapy for HCC. A higher level of BMP-7 in HCC cases compared to adjacent non-cancerous counterparts was reported [7]. Tacke et al. found an elevated serum level of BMP-7 in patients with cirrhosis as compared to non-cirrhotic patients [22]. Our data imply a correlation of increased liver damage with overexpression of BMP-7 in hepatocytes, as has also been reported previously [23, 24]. BMP-7 levels were also reported to be increased in animal models of liver cirrhosis [25]. Zoheiry et al. [13] observed elevations in BMP-7 levels with progression of fibrosis. BMP-7 serum levels were elevated in patients with chronic liver disease, and they were significantly higher in patients with liver cirrhosis compared to chronic hepatitis C (CHC) and HCC cases [13]. Overexpression of BMP-7 was reported to suppress liver fibrosis in rats [26]. On the other hand, the expression of BMP7 was found to be significantly down-regulated in HCC tissues compared with paracarcinoma tissues [27]. A possible explanation might lie in the modulatory balance of BMP-7 and TGF-β or another antagonist or inhibitor of the BMP-7 modulatory pathway. It was discovered that blocking BMP-7 signaling promoted invasive development of HCC cells [15]. Regarding the impact of the BMP-7 (rs230205) SNP on gene expression, it was observed that cirrhotic and HCC patients showed a significant elevation of BMP-7 levels with GG and AG carriers. Consequently, we supposed that overexpression of BMP-7 might be associated with the presence of the G allele. Concerning the progress of the disease, HCC patients showed a significant reduction in BMP-7 levels in HCC patients carrying the GG genotype compared to cirrhotic GG carrying patients. Therefore, the GG genotype might be correlated with the BMP-7 decline with disease development. Nevertheless, the current study should be viewed in the light of some limitations. The number of patients enrolled was relatively small. Further studies with a larger sample size are needed to confirm our results. In addition, we only examined the BMP-7 (rs230205) SNP. More SNPs in BMP-7 are needed to clarify the possible molecular mechanisms underlying progression of the disease.
Conclusions
Our pilot study explored the potential role of the BMP-7 (rs230205) gene polymorphisms in susceptibility to HCC in Egyptian patients. We found no significant difference in the distribution of all genotypes and alleles in the rs230205 (A/G) SNP between cirrhotic patients, HCC patients and controls. Collectively, this SNP might be considered a risk factor for cirrhosis and HCC with no significant association between the SNP and the progression of HCC from cirrhosis. We recommend additional prospective large-scale research to further confirm our findings.