Introduction
Acute Myeloid Leukaemia (AML) is a haematopoietic malignancy in which undifferentiated myeloid precursors arising from the bone marrow’s stem cells are clonally expanded at an early stage as they multiply, before infiltra- ting the peripheral blood and other organs [1–3]. It mainly affects the elderly population, with an average age of onset of 60–80 years, but it can also affect children. Paedia-tric AML constitutes about 25% of paediatric leukaemia, with an incidence rate of 7 per million children per year [3].
There is no gender difference in the occurrence rate, and disease rates are also similar among different racial groups [2–4].
Survival rates of between 60 and 70% have been reported, particularly due to the significant improvement in the prognosis of paediatric AML over the last 3 decades. These improvements are due to enhanced therapeutic approaches in induction chemotherapy, the consolidation process, the maintenance post-remission, and the transplantation of haematopoietic stem cells if necessary [5].
Treatment of refractory and relapsed AML in children, however, remains a therapeutic challenge, and the prognosis of recurrent disease is very poor. Relapse occurs in 24–40% of diagnosed children, and the long-term survival rate is approximately 30% [6].
The study aims to summarise the outcome of patients with refractory and relapsed AML, who were treated according to the Protocol Acute Myeloid Leukaemia Berlin-Frankfurt-Munster 2012 within the Polish Paediatric Leukaemia The Polish Paediatric. Leukaemia and Lymphoma Study Group institutions as their first-line therapy. The authors also aim to highlight the future perspectives on the treatment of relapsed AML in children, based on current research.
Patients
The group consists of 10 patients with primary refractory AML, and 30 patients with a first-time relapse of AML. All 10 patients with primary refractory AML were stratified as hazard ratio AML in their first diagnosis. The characteristics of the sex and age of patients are shown in Table 1.
Table 1
Characteristic features of patients with primary refractory acute myeloid leukaemia and relapsed acute myeloid leukaemia
| Parameters | Refractory AML (n = 10) | Relapsed AML (n = 30) | |
|---|---|---|---|
| Sex | |||
| Male | 2 | 21 | |
| Female | 8 | 9 | |
| Age (months) | |||
| Mean age | 9.2 | 11,9 | |
| Median age | 9.5 | 12.0 | |
| Minimum | 0.6 | 3.1 | |
| Maximum | 17.5 | 23.5 | |
The characteristic features of relapsed AML patients are shown in Table 2. The median time from the first diagnosis to relapse was 9.52 months (min 1.60, maximum 45.50 ±9.44).
Material and methods
The qualitative data are presented as N (%). The quantitative data are described using mean, median, maximum, and minimum values. To describe survival characteristics, the Kaplan-Meier curve was used. The log-rank test for the comparison between the 2 survival curves was also used. Statistical analysis was performed using MedCalc® Statistical Software version 20.027 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2022).
Results
Primary refractory acute myeloid leukaemia
Two out of 10 patients (2/10) died before re-induction therapy was started. One patient died 2 months after the diagnosis of relapsed AML; the cause of death was progression of the disease, sepsis, and fungal pneumonia. The second patient died 8 months after the diagnosis of relapse; the reason was also progression of disease and sepsis.
Re-induction treatment was started in 8 patients (8/10). The type of chemotherapy used in all 8 patients is described in Table 3. Complete remission (CR) was achieved by 3 patients (3/8; 37.5%).
Table 3
Reinduction treatment is used in patients with primary refractory acute myeloid leukaemia
| Treatment | n |
|---|---|
| IDA FLA + FLA | 3 |
| IDA FLA + FLA + GO | 2 |
| IDA FLAg + FLA + Clofarabine | 1 |
| IDA-FLA + FLA + TVTC + Bortezomib | 2 |
Allogeneic haematopoietic stem cell transplantation (allo- HSCT) was performed in 5 patients (5/8; 62.5%) – 3 patients (3/5) were transplanted in CR, and in 2 patients (2/5) the procedure was performed without remission of leukaemia. One of the 10 patients (1/5) is still alive, who has stayed for 34 months in CR since the allo-HSCT procedure (last follow-up in November 2021). The probability of 3-year event-free survival (pEFS) in this group is 0.125 ±0.11. ±0.11. Summary of outcome of primary refractory acute myeloid leukemia is presented in Table 4.
Relapsed acute myeloid leukaemia
Two patients (2/30) died before the re-induction treatment was started – the first one due to complications of the treatment, and the second due to progression of the disease.
In 2 patients (2/30), the remission has not been assessed yet because the follow-up was performed during the beginning of their re-induction treatment.
Twenty-six patients (26/30; 86.6%) underwent re-induction treatment – most of them (11/30) with the IDA-FLA Protocol (Fludarabine, Idarubicin). Second complete remission (CRII) was achieved in 9 patients (9/26; 34%), 8 of whom (8/9) underwent allo-HSCT in CRII, and one patient (1/9) had a second relapse.
Second complete remission was not achieved in 17 patients (17/26; 65%); allo- HSCT was performed in 7 of them (7/17), and 10 of them (10/17) died due to progression of the disease.
In total, 15 patients underwent allo-HSCT, and a summary of their outcomes is shown in Table 5.
Table 5
Summary of outcome in relapsed acute myeloid leukaemia patients, who underwent allogeneic haematopoietic stem cell transplant
Out of 11 patients with relapsed AML who were not treated with allo-HSCT, 10 patients passed away due to progression of the disease, and one patient relapsed for a second time.
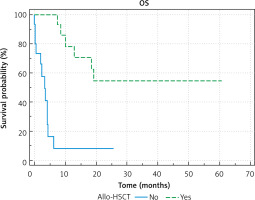
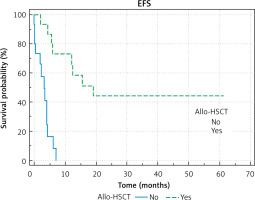
The survival in the relapsed group of patients was: pEFS = 0.24 ±0.08; probability of overall survival = 0.34 ±0.09. Better results were observed in patients who underwent allo-HSCT: OS 0.54 ±0.14 vs. 0.08 ±0.08, p < 0.0001, pEFS 0.44 ±0.13 vs. 0 ±0.00. Figures 1 and 2 show the difference between OS and EFS of patients treated with and without bone marrow allotransplantation procedures.
Discussion
Refractory and relapsed AML in children have very poor outcomes, and no specific guidelines for the treatment of these states currently exist. Prognostic factors that contribute to more favourable outcomes include an early response with CR after second-line treatment, late relapse, no previous allogeneic HSCT, and initially favourable cytogenetics [6].
It is already known that allogeneic HSCT as a second-line treatment improves the prognosis of children with both refractory and relapsed AML, especially in those who achieved complete remission [7]. In our study, the results of treatment of patients with refractory AML were extremely poor; only one patient survived, and his therapy included allo-HSCT in CRI. The probability of OS in the relapsed AML group was significantly higher in transplanted patients than in patients treated without allo-HSCT – better results were observed in patients who underwent allo- HSCT: OS 0.54 ±0.14 vs. 0.08 ±0.08, p < 0.0001. Within transplanted patients, a better outcome was observed in patients who achieved CRII before the allo-HSCT procedure (5/8 patients survived versus 2/7). Despite a small number of patients, our data can confirm that allo-HSCT in CRII is a potential curative method of treatment, although it can be less effective in patients who did not achieve CRI. The worst effect of allo-HSCT in patients without CRII has already been observed in other studies [8, 9]. This indicates the need for a possible therapeutic agent other than intensive chemotherapy.
There have been several perspectives of targeted therapies in AML including drugs that are more specific in targeting the leukaemic cell, thus resulting in remission.
According to Lonetti et al., Nivolumab is a programmed death-ligand 1 used to treat many cancers such as non-small-cell lung cancer, melanoma, and renal cancer. A remission rate of 18% was obtained in elderly patients with some mild side effects [2, 10].
This study will be the first to test the effects of Nivolumab in combination with 5-azacytidine (AZA) in paediatric patients with haematological malignancies.
Nivolumab has gone through a phase I/II study combination with AZA in paediatric patients with relapsed/refractory AML. The actual study start date was 29 November 2019, with a primary completion date of 30 March 2022, and it is estimated to be completed on 30 March 2024. Ages eligible for the study are 1–30 years, so it includes children and adults. Patients will receive the first dose of nivolumab on day 1 along with AZA. On day 15, the second dose of nivolumab will be given, enhancing its effect on the regenerating CD4+ and CD8+ memory T-cells [2, 10, 11].
Fms-like tyrosine kinase 3 inhibitors
Fms-like tyrosine kinase 3 (FLT3) is a protein on the surface of leukaemic cells that usually sends signals to cells to multiply. When mutated, it can drive the pathway causing the cells to multiply faster and resist apoptosis. Fms-like tyrosine kinase 3 mutations are the most common mutations that develop in AML patients (30%) [12].
Fms-like tyrosine kinase 3 inhibitors consist of 2 generations – the first generation includes midostaurin and sorafenib, and the second generation includes quizartinib, gilteritinib, and crenolanib. The second generation is more potent and specific than the first, inducing up to 40% remission in relapsed AML [13].
Gilteritinib, known by the trade name Xospata, is a selective FLT3 inhibitor used in relapsed/refractory FLT3 mutated AML. It has been shown and proven that it enhances remission and survival. This drug is the first approved FLT3 inhibitor to be used alone as monotherapy. In 2018, the European Medicines Agency approved modification of the paediatric investigation plan of gilteritinib, led by Astellas Pharma Inc. (Astellas Pharma Global Development, Inc.), “A Study of Gilteritinib (ASP2215) Combined With Chemotherapy in Children, Adolescents, and Young Adults With FMS-like Tyrosine Kinase 3 (FLT3)/Internal Tandem Duplication (ITD) Positive Relapsed or Refractory Acute Myeloid Leukaemia (AML)”. The study consists of phase I (dose escalation) and phase II (dose expansion). One cycle includes 28 days of treatment. The start date of this study was 27 February 2020, the estimated primary completion date is 30 April 2024, and the estimated final study completion is to be on 30 November 2028. The ages of patients that are eligible for the study were from 6 months to 21 years, so it included both children and adults [2, 14].
Quizartinib is an experimental drug, used only in medical research. It was evaluated in relapsed childhood AML combined with salvage chemotherapy. Cooper et al. stated that quizartinib resulted in “a favourable toxicity profile and an encouraging response, consisting in complete FLT3 inhibition in all patients, and 4/17 and 10/17 complete remissions or stable disease, respectively” [15].
Currently, quizartinib is being evaluated in a phase 1/2 study for its combination with re-induction chemotherapy and for use as mono-maintenance therapy in relapsed/refractory paediatric AML with FLT3-ITD mutations. According to the website “ClinicalTrials.gov”, the trial will be conducted in multiple phases. An independent data monitoring committee will protect the rights, safety, and well-being of participants by monitoring the progress and results. The data monitoring committee will comprise qualified physicians and scientists who are not investigators in the study and are not otherwise directly associated with the sponsor, and this will be convened at the end of Phase 1. The ages eligible for this study are between 1 month and 21 years. The actual study start date was 15 August 2018, and the estimated completion date is 1 May 2027 [2, 15, 16].
Isocitrate dehydrogenase inhibitors
Isocitrate dehydrogenase (IDH) is an enzyme in the Krebs cycle that helps cells produce energy in the form of ATP [17]. It is mutated in ~15–20% of AML patients, wherein this mutated IDH produces an abnormal product (2HG; hydroxyglutarate) and enters the nucleus. 2HG generally causes the cell to differentiate into normal white blood cells, red blood cells, and platelets, but when mutated, this mechanism blocks differentiation. Enasidenib and ivosidenib (designer drugs) selectively target mutated IDH [18].
Programmed cell death (apoptosis)
Apoptosis is made up of a series of reactions in the cell leading to cell death. BCL-2 is one of the proteins that mediate apoptosis. In AML, BCL-2 is overexpressed, which prevents cells from dying. Venetoclax is a potent and specific inhibitor pill that, once used by itself, leads to 20% complete remission, but when used with chemotherapy, the effect is synergised, promoting cell death [19]. Karol et al. used venetoclax in children and young adults with relapsed AML. They found that combining venetoclax with high-dose cytarabine in addition to or in the absence of idarubicin was efficient and well-tolerated. As a result of the first cycle of therapy, 14 (70%) out of 20 evaluable patients were able to achieve a complete remission or a complete remission with incomplete count recovery. Consequently, 10 (71%) of these 14 patients tested negative for measurable residual disease (MRD) [20].
Chimeric antibody receptor immune cells therapy
Chimeric antibody receptor (CAR) immune cell (T-cell) therapy is one of the main strategies being explored for AML despite its usage in ALL and lymphomas. In this type of treatment, the patient’s T-cells are withdrawn from the blood and infected with CAR to detect and recognise leukaemic cells (Ex: CD19 for B-cell acute lymphoblastic leukemia (B-ALL), then they are expanded and finally re-infused into the patient’s blood. In addition, CAR T-cells also penetrate the cerebrospinal fluid. This therapy has certain side effects leading to neurological symptoms and cytokine release syndrome [16]. However, compared with standard chemotherapy in relapsed ALL in children, it leads to fewer systemic toxicities. Due to several successful trials with CD 19-directed CAR T-cell treatment in childhood and young adults with ALL, several trials are being performed to achieve that success in AML. The problem is that AML cells lack AML-specific Ag and the Ag in most myeloid cells is co-expressed in normal haematopoietic stem cells and or in progenitor cells. In the article written by Willier et al., the main goal was to explore immunotargets in paediatric AML in comparison with adult AML. In their study, 36 bone marrow samples were taken from paediatric patients with AML undergoing whole RNA sequencing and flow cytometry. After several attempts, they were able to identify 2 targets on the cells: CD33 and CLEC12A. They found that these 2 targets were poorly expressed in healthy tissues compared with the other targets. Thus, there is hope in finding treatment using immunotherapy for paediatric patients with AML [21].
Bispecific tumour-engaging antibodies and mesothelin
Bispecific tumour-engaging antibody (BiTE) is a combination of 2 antibodies linked together, which binds to the surface of tumour cells and induces the body’s immune T-cells to recognise, attack, and kill tumour cells. This technique is considered a direct pathway used in B-ALL with 2 complete cases of remission, but similar monoclonal antibodies treat AML by targeting different proteins on the AML blast cells [22]. Mesothelin (MSLN) is a protein normally found in tissues lined by mesothelial cells such as the pleura, peritoneum, and pericardium. It is known to be a cell surface marker overexpressed in several solid tumours such as adenocarcinomas. The function of mesothelin is still unclear, but it is said to be involved in the cell adhesion process in normal and malignant cells [23].
An article published by Gopalakrishnapillai et al. was the first to show that immunotherapy using MSLN-targeting bispecific antibodies is viable for MSLN-positive paediatric AML [24]. Mesothelin is considered to be a viable target for immunotherapy because it is not expressed in normal bone marrow cells. In the beginning, only MSLN was thought to be overexpressed in some adult malignancies, but with research, scientists found that MSLN is only one of 7 genes to be overexpressed in AML paediatric patients, specifically in their bone marrow cells [25].
Clinical trials have been performed in mice by using BiTE antibodies targeting MSLN, by the fusion of a domain in amatuximab with CD3, targeting another domain from blinatumomab, in order to check whether this immunotherapeutic approach could be successful. The trials showed that this targeting is successful, by prolonging survival and decreasing the leukaemic expansion in bone marrow cells [24].
Measurable residual disease assessment
Prognostic assessment of AML at diagnosis is very important because it allows us to qualify patients to a high-risk group and decide whether the treatment should be enhanced with allo-HSCT. Next-generation sequencing (NGS) technologies enable the detection of novel molecular abnormalities. This method can also identify potentially important changes that occur at the sub-clonal level during the disease course, for example, changes in FLT3-ITD mutations [25]. For this reason, the updated European LeukemiaNet recommendations include the definition of MRD-negative molecular complete remission in cases where a genetic or immunophenotypic aberration that was present at diagnosis bur was not detectable by high-sensitivity multiparametric flow-cytometry or reverse transcriptase-quantitative polymerase chain reaction [26]. The question now is whether the NGS technology should also be included in paediatric AML protocols, and if so, at which point of treatment would this very sensitive method be helpful to identify patients with worse outcomes [27].
Conclusions
The prognosis of refractory AML and the first AML recurrence in children who were first-line treated in Polish Paediatric Group for Treating Leukaemia and Lymphomas centres according to Acute Myeloid Leukaemia Berlin- Frankfurt-Munster 2012 protocal is unfavourable. Failures of re-induction treatment still result from difficulties in achieving sustained remissions, and to a lesser extent from deaths due to complications. Allogeneic HSCT improves the prognosis in children with refractory and first recurrent AML, under the condition that they achieve complete remission. A great deal of research concerning novel agents gives hope that the outcome of recurrent and relapsed AML in podiatric patients will improve soon. A very accurate assessment of the MRD is needed to adjust the proper therapeutic path and enhance the therapy if needed, aiming to avoid the recurrence of the disease.