Introduction
A new category of biomarkers, which has attracted growing interest in the screening of several tumors, including lung, breast, prostate, renal and colorectal cancer (CRC), is circulating tumor cells (CTCs) [1–3]. These tumor cells can be isolated from the peripheral blood circulation. Their enumeration may potentially predict survival and response to treatment in patients. This review, based on the literature findings, aims to highlight the clinical utility of CTCs in CRC patients and the detection methods.
Colorectal cancer
Colorectal cancer is one of the most common cancers and the second cause of cancer deaths worldwide [4]. Since no early screening procedure has been standardized yet, CRC is mostly diagnosed at relatively advanced stages, making it difficult to properly treat. Worldwide, 95% of CRC cases are sporadic and 5% are hereditary [5]. Colorectal cancer is localized in the colon or the rectum. About 90% of CRCs are adenocarcinomas; the remaining 10% consist of rare forms of CRC that may contain lymphomas and sarcomas [6].
Risk factors for development of CRC can be clinical, older age, previous colonic polyps, and presence of inflammatory bowel disease [7, 8]. Environmental factors play a major role in developing CRC: sedentary lifestyle, consuming red meat, obesity, smoking and alcohol consumption [9–11].
Screening for CRC includes fecal occult blood testing, flexible sigmoidoscopy and colonoscopy, and it should be done every ten years to prevent development of polyps along with the dosage of serum levels of carcinoembryonic antigen (CEA); a high level > 5 ng/ml is significantly associated with shorter overall survival (OS), and carbohydrate antigen 19-9 is considered a serum marker for liver metastasis [12, 13].
In order to manage CRC, clinicians consider several factors to predict a patient’s prognosis. First of all are clinical factors such as tumor-node-metastasis (TNM) stages [14]. Furthermore, histological features include mainly the type of the tumor, which is adenocarcinomas [15]. Moreover, genetic factors include a high frequency of chromosomal instability and microsatellite instability (15% of CRCs), mutations in codons 12 and 13 responsible for the expression of gene Kirsten rat sarcoma viral oncogene homolog (KRAS) (40% of CRCs), mutation in BRAF (10% of CRCs) and alteration of tumor suppressor genes, for instance TP53 [12, 14, 16–19].
Treatment of CRC patients depends on the stage of the tumor, according to which the patients are either eligible to undergo a surgical procedure to ablate the tumor or benefit from chemotherapy if the tumor is difficult to resect [20]. However, treatment options seem to be of limited efficiency if the tumor has already metastasized. The last decade witnessed the rise of a new screening method utilizing tumor cells disseminated in the peripheral blood to prevent inducing metastasis and eventually increasing the overall survival. Those cells are the circulating tumors cells.
Circulating tumor cells
The two main biomarkers detected in liquid biopsies are CTCs and circulating tumor DNA (ctDNA), and both provide complementary information. But the analysis of ctDNA remains limited, since the latter is difficult to detect in comparison with CTCs; it is fragmented and found in small concentrations [21]. Furthermore, ctDNA’s role is also limited; it can only be analyzed at the genomic level, unlike CTCs, which can be analyzed at genomic, transcriptomic and proteasomic levels using in vivo (isolated and enriched CTCs implanted in animal models) and in vitro functional assays [21]. That is why we chose to focus on CTCs rather than ctDNA in our review.
Isolation of CTCs provides the possibility of studying them in terms of morphology and phenotyping through to molecular testing, enabling comparison with the primary tumor, which is not the case for ctDNA. CTCs also offer an accessible, non-invasive source of tumor material from cancer patients.
Circulating tumor cells are tumor cells shed in the blood circulation by the primary tumor. Tumor cells can infiltrate as single cells, in clusters, in strands or in single files [22]. Ashworth first described these cells in 1869; cells similar to those found in tumors were found in the blood after death of the patient [23]. However, in the last decade the research field took interest in these cells and their massive role in prevention of metastasis and relapse.
Millions of tumor cells are released in the circulation but only some of them can reach their destination and induce metastasis in distant sites (Fig. 1) [22]. This decrease of CTC number is due to the fact that CTCs are affected by several mechanisms which prevent them reaching the distant organs. Those obstacles are shearing forces and collisions with blood cells, generated by the blood flow. Circulating tumor cells that have undergone epithelial-mesenchymal transition (EMT) can resist these forces [24]. Epithelial-mesenchymal transition is a phenomenon whereby CTCs change from the epithelial phenotype via downregulation of expression of the epithelial markers (Fig. 2) to the mesenchymal phenotype by upregulating its markers (Fig. 2) during the metastatic process in order to acquire migratory ability and be invasive. Colorectal carcinomas use EMT in order to initiate invasion via activation of the following signaling pathways: transforming growth factor, Wingless/ integrated WNT, platelet-derived growth factor and IL-6 [25]. This leads to activation of the following transcription factors: SNAIL, TWIST and Zinc Finger E-Box Binding Homeobox responsible for the mesenchymal phenotype [22].
Fig. 1
Circulating tumor cells are tumor cells released into the circulation from the primary tumor. Their transendothelial passage is facilitated by the epithelial-mesenchymal transition phenomenon. In contrast, tissue colonization of a metastatic site is accompanied by a mesenchymalepithelial transition phenomenon, and the tumor cells thus extravasated regain their epithelial phenotype
The figure was created using BioRender.com (accessed on 4 December 2022).
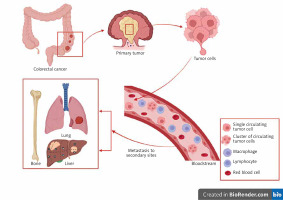
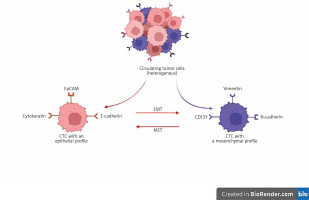
Fig. 2
Epithelial and mesenchymal markers of circulating tumor cells
The figure was created using BioRender.com (accessed on 4 December 2022).
CTC – circulating tumor cell, EMT – epithelial-mesenchymal transition, EpCAM – epithelial cell adhesion molecule,
MET – mesenchymal epithelial transition
Anoikis is when cells lose their attachment to the extracellular matrix and surrounding cells resulting in their apoptosis. Resistance to anoikis is achieved by activating tropomyosin-related kinase B, which may allow cancer cells to survive during systemic circulation, promoting subsequent tumor growth in distant organs [26].
On the other hand, CTCs may escape the immune system by developing a protecting phenotype with the overexpression of CD47 in CRC. This marker is anti-phagocytic and is expressed by the tumor cells to prevent their attack by macrophages and dendritic cells [27].
Circulating tumor cells are rare in the peripheral circulation, which makes their detection challenging. Adding to that, the most commonly used techniques to isolate CTCs target only their epithelial phenotype; so, CTCs that have undergone EMT may not be identified.
Markers
The characterization of CTCs in CRC is based on the expression of CTC surface antigens. These antigens may be epithelial markers such as epithelial cell adhesion molecule (EpCAM) and cytokeratins (CK). However, due to the EMT the dedifferentiated CTCs may also express mesenchymal markers such as vimentin or N-cadherin (Fig. 2). This transition makes their capture quite challenging.
EpCAM
The epithelial cell adhesion molecule is a cell surface glycoprotein of 40 kDa, overexpressed in epithelial cancers, which is often associated with decreased patient survival. The epithelial cell adhesion molecule is involved in cell proliferation, migration, adhesion, differentiation and cell signaling. High expression of EpCAM usually correlates with poor prognosis except in renal and thyroid carcinoma, where its overexpression correlate with increased survival [28]. A Chinese meta-analysis suggested that EpCAM expression is higher in CRC patients than in normal controls. However, loss of its expression correlates with the progression, metastasis and poor prognosis of CRC [29].
Cytokeratins
Cytokeratins are epithelium-specific intermediate filaments with diameters of 6 nm to 25 nm [30]. They are an important component of intermediate filaments, which help cells resist mechanical stress due to their flexibility and ability to stretch. Circulating tumor cells expressing CK (8, 18, 19) reveal their epithelial origin. Cytokeratin 20 is a well-known cytokeratin expressed mostly by gastrointestinal epithelial cells, subsequently by the tumor derived from these cells [31]. Hinz et al. found a significant correlation between CK 20 expression and worse 5-year OS and disease-free survival (DFS) [31].
N-cadherin
N-cadherin is a transmembrane protein; it is expressed abnormally and plays a role in cancer metastasis by providing a mechanism for transendothelial migration. In CRC, N-cadherin may be an independent prognostic marker since its high expression was correlated with tumor progression [32]. Targeting N-cadherin helps isolate CTCs that have undergone EMT [33].
Vimentin
Vimentin is a mesenchymal marker, which belongs to the type III intermediate filament family. It plays a massive role in the immune response, stabilizing cytoskeletal interactions and preserving the cell shape. In CRC, expression of vimentin is correlated with a poor prognosis of the disease result [34]. In prostate cancer, vimentin was used to enumerate CTCs with a mesenchymal origin and was associated with cancer progression [35].
Methods of detection
Several techniques have been established to enumerate and characterize CTCs from blood circulation in CRC (Table 1). Circulating tumor cells are rare (1–100 cells per ml), which represents a challenge for their isolation [36].
Table 1
Different techniques used for circulating tumor cell isolation, enrichment and characterization in colorectal cancer
| Technique | Description | Ref. | |
|---|---|---|---|
| CTCs‘ isolation and enrichment | Immunomagnetic separation | ||
| CellSearch® | Enrichment of CTCs is achieved by using ferrofluids coated with EpCAM antibodies. Every tumor cell with a profile of EpCAM+, CD45- and 4',6-diamidino-2-phenylindole+ is considered a CTC. This technique is the only one approved by the Food and Drug Administration although it remains limited to isolation of epithelial tumor cells only | [1] [37] | |
| Adna test | Enrichment of CTCs is achieved by using magnetic beads coated with EpCAM and mucin 1 antibodies, in breast and colorectal cancer | [38] [39] | |
| Size based separation | |||
| Isolation by size of epithelial tumor cells | This technique was developed by Giovanna Vona and her team in 2 000. It provides morphological, immunocytological and genetic characterization of CTCs. A small amount of blood is filtered. This filtration allows blood cells to pass and contain CTCs due to their larger size | [40] | |
| Screen cell | The blood flow passes through a microporous membrane filter attached to a metal ring placed between a filtration reservoir and a detachable nozzle which guides the insertion of a collection tube to vacuum the blood through an IS leaving the CTCs on it | [16] [41] | |
| Density based separation | |||
| Density gradient centrifugation/CellBlock | After centrifuging diluted blood with Ficoll-Paque, isolation of CTCs is done from the buffy coat that contains peripheral blood mononuclear cells. Then, the cell suspension will be subjected to: CytoSpin to finally obtain slides of CTCs fixed by ethanol and ready for characterization Construction of a cellblock with solidifying agents such as gelatin or HistoGel | [42] [43] | |
| Rosettesep | This assay is the only EpCAM-independent enrichment method to detect CTCs. It is based on negative enrichment by leukocyte depletion. Unwanted cells are crosslinked to red blood cells forming immunorosettes | [44] | |
| Microfluidic technologies | |||
| CEE technology | CEE is a platform enriching rare cells by combining antibody-functionalized surfaces with a microfluidics channel. Using functionalized posts and particular antibodies that only adhere to target cells, the CEE system captures cells as they pass past it. Cells that have been captured can be used for additional research. The cell sample remains suitable for characterization | [45] | |
| CTCs’characterization | Immune approaches | ||
| Immunocytochemistry | This technique is used to characterize the isolated CTCs. It is used to study the expression of a specific protein or antigen in CTCs. The target antigen is combined with a specific primary antibody. Then the reaction can be visualized by an amplification/revelation system | [46] | |
| EPISPOT | The EPISPOT plate is composed of a nitrocellulose membrane precoated with a specific antibody targeting CTCs | ||
| DNA and RNA approaches | |||
| Multiplex PCR | After DNA extraction, multiplex PCR consists of using polymerase chain reaction to amplify numerous different DNA sequences at the same time. Thus, many genes can be targeted on the CTCs. More than one pair of primers is required in the same reaction. The primers can specifically combine with their corresponding DNA template, and more than one DNA fragment will be amplified in one reaction simultaneously | [39] [47] [48] | |
| RT-PCR | After RNA extraction, RNA is reverse transcribed into complementary DNA. Then, specific DNA target mutations are amplified using PCR. This technique is characterized by its high sensitivity and specificity. It gives the opportunity to study the mutations in CTCs | [31] | |
| RT-qPCR | Quantitative reverse transcription PCR (RT-qPCR) is used when the starting material is RNA. The cDNA is then used as the template for the qPCR reaction. The latter enables monitoring of the amplification in real time. This technique offers the possibility to profile the gene expression of the isolated CTCs | [49] [48] |
Some techniques are preferred to others, especially the immunomagnetic ones due to their sensitivity to detect CTCs using EpCAM antibodies.
Isolation and circulating tumor cell enrichment
Size based isolation
Isolation by size of epithelial tumor cells (ISET) and ScreenCell are two methods for isolating CTCs from whole blood based on their size and membrane capacity. Being larger than the other blood cells, CTCs are promptly isolated (Table 1).
Isolation through immunoaffinity
Targeting the epithelial markers such as EpCAM is one of the assays used to select CTCs positively, e.g. CellSearch, Adna Test. These techniques use immunomagnetic beads coated with EpCAM antibodies. Other immunoassays proceed by CD45 expressed cell depletion such as epithelial immunospot (Table 1).
Density based separation
Density gradient centrifugation of diluted blood allows the enrichment of CTCs from the buffy coat containing peripheral blood mononuclear cells (PBMCs) (Table 1).
Characterization of circulating tumor cells
After isolation and enrichment of CTCs, their characterization may be done through immunoassays such as immunocytochemistry or using molecular approaches (Multiplex PCR, RT-PCR, RT-qPCR) (Table 1).
The detection and utilization of CTCs in a routine clinical setting are cost-effective because conventional patient management and monitoring techniques are either time-consuming or very costly. And since one of the major advantages of CTCs as a liquid biopsy is the possibility of repeated analyses, it would be economically challenging to implement them in cancer management unless further advances are made in reducing the cost of conventional techniques while increasing their effectiveness.
Furthermore, there are several limits in the detection of CTCs regarding some techniques, especially those targeting epithelial markers of CTCs, which are suitable only for well-differentiated cancer cells but not for that undergoing the epithelial mesenchymal transition. Other limitations are viability of CTCs after their detection, the fact that techniques using immunomagnetic CTC enrichment by antibodies are less sensitive in the early stages of tumor development, and approaches using immunomagnetic beads coated by antibodies present a possibility of false-positive findings due to expression of a selection marker being present in other cells [50]. Other techniques are size dependent, like ISET; it cannot discriminate between malignant and benign cells and the diameter of the pores may allow small tumor cells to pass through, making this approach less sensitive [51]. In molecular approaches there is no morphological confirmation of the isolated CTCs [50].
Clinical utility of circulating tumor cells in colorectal cancer patients
Circulating tumor cells represent a new category of bio-- markers; these tumor cells play a massive role in the screening of several tumors including lung, breast and prostate cancer [1, 38, 52]. Isolating CTCs has been the aim of numerous studies as well as associating their count with the histopathological features and predicting the outcome of the disease and the response of the patients regarding the treatment.
As a serum biomarker for CRC, it was crucial to study whether there is an association between CEA and CTCs. A significant correlation was reported between high CTCs at baseline and the rate of CEA along with a high tumor/liver ratio [53]. According to Aggarwal et al., associating CTCs with CEA might add predictive information in terms of prognosis: a cut-off of CTCs ≥ 3/7.5 ml with CEA < 50 ng/ml predicts a decrease in survival [54]. A study conducted by Yang et al. suggested that the combination of CTCs with the tumor marker CEA has better OS prediction than individual CTCs or CEA and serves as a more effective prediction model in patients with CRC [55].
Cytokeratins 20 is a well-known CRC biomarker. In colon cancer patients, presence of CTCs which express CK20 is associated with a significantly higher risk of developing a recurrent disease compared to the group without CTCs [31].
In a study aiming to highlight the clinical relevance of EMT in CTCs from metastatic CRC patients, those who do not express CK20 may express phosphoinositide 3-kinases (PI3Ka) and protein kinase B (Akt2), whose expression has been implicated in EMT and cancer stem cell renewal [48].
Correlation between CTC count and TNM stages was the aim of Eliasova’s study conducted on 98 patients. Circulating tumor cells were detectable in all the stages with high percentages especially in Stage II (92.86%) although this result was statistically non-significant [56]. In contrast, in a prospective study conducted on 287 non-metastatic CRC patients, the association between CTCs and tumor stages was significant [57]. Patients with a stage IV CRC and a diffuse metastasis had a high number of CTCs compared to those with a limited metastasis to lung or liver with a low number of CTCs [58].
Patients suffering from a non-metastatic CRC and with a high CTC count (> 5 CTCs/2 ml) may develop distant metastasis in contrast to patients with a CTC count < 5. Thus CTCs may be considered as an independent prognostic marker for non-metastatic CRC patients with high risk of early recurrence [59]. This conclusion is in concordance with Thorsteinsson et al., who found a low rate of CTCs in non-metastatic colon cancer and it decreased post-operatively [60].
Lung and liver are the main sites of metastasis in CRC patients. A peak of CTCs was observed after the resection of the metastasis [61]. Furthermore, they found that CTCs express macrophage and leukocyte markers such as CD14 and CD45 so they hypothesized that CTCs can fuse with macrophages so that they can be tolerated by the immune system [61]. Recently, these cells have been described as circulating hybrid cells. They are also used as biomarkers for cancer diagnosis and monitoring; indeed dual expression of CK and CD45 was reported to be associated with a worse prognosis [62].
Among the genetic factors of developing CRC, there is KRAS mutation. To determine the origin of the CTCs and demonstrate that they are colorectal tumor cells, it was crucial to research the KRAS mutation in CTCs. Buim et al. found concordance between CTCs and the primitive tumor in term of KRAS mutation [40]. Adding to that, the presence of KRAS mutation is a specific predictive marker for inefficacy of cetuximab, which is a monoclonal antibody that specifically blocks epidermal growth factor receptor [63].
The association between the progression of CTCs during the treatment and the relapse was significant (p = 0.029) in the study carried out by Garrigós et al. [64]. A high number of CTCs predict a decrease in OS in advanced CRC patients receiving cetuximab as a third line treatment combined with chemotherapy [63]. The screening of CTCs in the follow-up may predict a decrease in the survival of the patients with a positive CTC status [65]. Even at an early assessment, CTC status during treatment was significantly associated with tumor response: patients who had an unfavorable CTC changed profile did not respond to the treatment. Therefore, these patients had a significantly shorter progression-free survival (PFS) and OS in contrast to those harboring a favorable CTC profile. Moreover, CTC status assessed early during treatment with an anti-EGFR monoclonal antibody may predict treatment failure in advance compared to imaging-based tools [39].
Predicting response to treatment has been the aim of numerous studies. A decrease in the rate of CTCs or their disappearance is correlated with the efficiency of the treatment in metastatic CRC patients, whereas their continuous appearance reveals progression of the tumor [38]. Enumeration of CTCs before and during treatment can be an independent prognostic marker for PFS and OS. Patients with a low number of CTCs during the first chemotherapy had a good outcome in contrast to those with a persistently high number of CTCs [66]. This study offers the possibility of molecular characterization of CTCs in order to create a personalized and targeted therapy [49]. A Spanish study carried on 180 metastatic CRC patients receiving first line chemotherapy plus bevacizumab showed that enumeration of CTCs before treatment is a strong and independent prognostic factor for the results of PFS and OS. Thus a change in the number of CTCs during treatment defined 3 groups: patients with a number below 3 have a long PFS and OS; patients with a high number of CTCs before and during treatment have a short PFS and OS; and patients in whom the number converted from high to low have an intermediate result [67]. A Chinese team studied the count of CTCs and the prognosis of patients after platinum-based first line chemotherapy: patients with presence of CTCs suffered from a short DFS after receiving the treatment (p < 0.01) compared to the patients from the CTC- group. They also found that a high count of CTCs was associated with high expression of vimentin, E-cadherin, RAS mutation, tumor location and tumor M-stage. They also suggested that targeted therapy with cetuximab might have worse efficacy in patients with a high CTC count [68].
The clinical potential of isolating CTCs in CRC patients has been shown by several studies; a pilot study conducted by Cohen et al. on 430 patients with metastatic CRC aimed to study the relationship of CTCs with tumor response, PFS and OS. They demonstrated that a number of 3 or more CTCs in 7.5 ml of blood was correlated with a decrease in PFS and OS whether it is at baseline or during treatment. This study was the first to set a cutoff of 3 CTCs, with CellSearch, which is associated with a worse prognosis [69]. Therefore, CTC analysis using CellSearch technology as routine procedure is applied in therapeutic management of metastatic breast, colorectal and prostate cancer patients. The same team found in another study that OS decreased statistically significantly in metastatic CRC patients with unfavorable CTCs, but when the patients recovered from unfavorable to favorable in terms of CTCs, consequently there was an increase of PFS and OS [70]. Also, Barbazan et al. demonstrated that before treatment, PFS and OS of the patients with a high level of the CTC markers was 6.3 and 12.7 months respectively against 12.7 and 24.2 months in patients with a low level of CTCs [71]. Li et al. found an association between the presence of CTC count and advanced age (≥ 63 years old; p = 0.037), a high platelet-lymphocyte ratio value (p = 0.008) and a high neutrophil-lymphocyte ratio value (p = 0.034). Also the CTC count was significantly associated with PFS of metastatic CRC patients who received chemotherapy [72].
Detection and enumeration of CTCs plays an outstanding role in predicting the prognosis and cancer management as well as in predicting a relapse in the future. Several recent studies have demonstrated that CTC count was an indicator of poor prognosis (Table 2).
Table 2
Prognostic/predictive value of circulating tumor cells in colorectal cancer
| Patients and CTC detection methods | CTCs (% of positivity or number) | Prognostic value | Ref. |
|---|---|---|---|
| 61 patients/CellSearch | 44.3% (27/60): at least one CTC was detected | Heterozygosity and heterogeneity in KRAS status amid CTCs compared to primary tumors | [73] |
| 50 patients/CellSearch | 64% of patients at baseline had detectable CTCs | A decrease in PFS in patients with ≥ 3 CTCs after treatment with regorafenib | [37] |
| 130 patients/MACS | 51.54% (67/130) | Postoperative CTCs is not only an independent factor in predicting tumor recurrence in stage II–III CRC, but also an indicator of poor prognosis when associated with female gender, older age, higher TNM stage, and preoperative CEA levels | [74] |
| 80 patients/GILUPI CellCollector, CellSearch | 31.3% (25/80) by CellSearch and 41.3% (33/80) by CellCollector | Presence of CTCs as an indicator of poor prognosis when correlated with advanced “Union for International Cancer Control” stage, distant metastasis, presence of lymph node metastases, and reduced overall survival | [75] |
| 57 patients/(44) NYONE, (31) ScreenCell, (41) Cytokeratin-20 RT-qPCR | 36.4% (NYONE), 100% (ScreenCell), 80.5% (PCR) | All methods revealed a positive correlation of CTC presence and higher tumor burden (advanced stages) | [41] |
| 589 patients/CellSearch | 41% (241): ≥ 3 baseline CTCs count/7.5 ml | ≥ 3 baseline CTC count as an indicator of worse prognosis when associated with worse performance status, stage IV at diagnosis, three metastatic sites (liver, bone, lung) and elevated CEA levels | [76] |
| 175 patients and 127 patients/CTCBIOPSY, Immuno-cytochemistry | 42.29% (74/175) pre-MCTC, 32.28% (41/127) post-MCTCs | Mesenchymal CTC status as a sign of poor prognosis when associated with unfavorable clinicopathological parameters such as presence of lymphovascular invasion and advanced TNM stage. The persistent positivity of mesenchymal CTCs before and after anticancer therapy was an independent risk factor affecting the OS and RFS of CRC patients | [77] |
| 40 patients/flow cytometry | ≥ 4 CTCs: 60 % (24/40) at T1 (skin incision), 72.5% (29/40) at T2 (after surgical resection) | Elevated CTC counts at T2 were significantly associated with female sex, vascular invasion, tumor localization in the colon and metastatic lymph nodes | [78] |
| 149 patients/immuno nano-magnetic spheres | 48.32% (72) > 3 CTCs/7.5 ml | Elevated CTC counts were associated with increasing tumor stage and T stage, negative changes in the survival curve and risk curve escalated more rapidly in the CTCs+ group | [79] |
| 186 patients/MiSelect R system | 37% (69) | CTCs count of ≥ 5 as an indicator of worse OS as well as poor prognosis when associated with lower CEA levels, higher CA19-9 levels, and both node status and presence of distant metastasis | [55] |
| 218 patients/CellSearch | 24% (51) at baseline, 38% (83) at the time of disease progression | Great prognosis observed in case of total CTC absence while their presence at any timepoint is associated with a poor prognosis (lower PFS and OS) | [80] |
Table 2 shows the prognostic and predictive value of CTC detection in patients with CRC. The table also shows values such as the number of patients, the methods used and the ratio of CTC positivity.
Conclusions
Through this review of the literature, we wanted to highlight all the aspects related to CTCs, namely the techniques of detection and characterization on the one hand, but also the role of these cells in the management of patients with CRC. This review was intended to be exhaustive in order to allow a useful and efficient adjustment of the undeniable role of this new type of markers in the management of CRC. CTCs are involved in the management of CRC in terms of therapeutic and prognostic management, including the need for CTC status in order to decide on the course of treatment.
This review highlights the clinical potential of CTCs in CRC patients. As a biomarker, CTCs play a major role in predicting the response of the patients regarding the treatment and a relapse in the future.








