Introduction
Otitis media with effusion (OME; secretory otitis media; glue ear) is an inflammatory condition of the middle ear that involves accumulation of sterile fluid, or effusion, in the tympanic cavity, with the tympanic membrane intact. This effusion in the middle ear is due to Eustachian tube obstruction, which generates relative negative pressure in the tympanic cavity. The fluid may be a transudate, an exudate, or a mucous gland secretion. The transudate results from a hydrostatic imbalance between blood vessel lumina and extravascular spaces. The exudate is a sign of inflammation and indicates an altered permeability of vascular walls. Finally, the thick, mucous secretion is produced by glands found in the ear’s lining, which thickens and undergoes metaplasia into multilayered glandular epithelium over time [1–3]. Predominant clinical symptoms are hearing loss and the feeling of fullness and fluid swishing in the ear. Otoscopy findings may vary and depend on the type of fluid behind the tympanic membrane. There may be tympanic membrane thickening with hyperaemia at its periphery and along the manubrium mallei, small bubbles of air or an air-liquid level visible behind the tympanic membrane, and/or an amber-coloured or bluish tinge to the tympanic membrane. OME is characterized by its seasonal character, with peak rates during fall and winter and lower rates during summer months. OME is usually diagnosed after an episode of acute otitis media and lasts about 40 days on average. Chronic OME is diagnosed when the condition persists for over 3 months or there are at least 6 episodes over a 12-month period [2, 3]. The key factor in OME pathogenesis is Eustachian tube dysfunction. A normally functioning Eustachian tube allows air to enter the middle ear during swallowing (which equalizes air pressures on both sides of the tympanic membrane); prevents pathogens from entering the middle ear from the nasopharynx; and drains the middle ear via mucociliary clearance. Eustachian tube impairment, either structural or functional, leads to inflammation of the middle ear [3, 4].
The pathophysiology of OME is undoubtedly multifactorial. The factors promoting OME development include age, male sex, attending a nursery, having older siblings, and a low birth weight. One important environmental factor is exposure to cigarette smoke. Inflammatory factors include recurrent upper respiratory infections, primary ciliary dyskinesia, chronic sinusitis, nasal polyps, inadequately treated bacterial acute otitis media, and adenoid hypertrophy. Anatomical factors (craniofacial developmental anomalies) include a cleft palate. In adults, there are also nasopharyngeal cancer, ear barotrauma, and radiation-induced ear toxicity [1, 3, 5]. Moreover, there has been intensive research into the role of allergies in OME pathophysiology over the last several years. According to the ‘one airway, one disease’ concept, suggesting that the entire airways may be affected by an allergic condition, an inflammatory allergic response may be expected to affect the middle ear as well. Importantly, the exudative fluid in patients with OME and a comorbid allergy contains all the cells and mediators found in allergic inflammation. Moreover, nasal mucosa stimulation by allergens leads to Eustachian tube oedema and inflammation, which results in tube dysfunction and development of OME [1, 5]. A cohort study by Kreiner-Moller et al. (Copenhagen Prospective Studies on Asthma in Childhood (COPSAC)) evaluated the relationship between atopic conditions and OME in children. That study demonstrated co-occurrence of OME and allergic rhinitis (OR = 2.29); however, it did not demonstrate any significant impact of non-allergic rhinitis, asthma, or eczema on the development of OME [6]. In 1984, Tomioka described a new, intractable type of otitis media, eosinophilic OME [1]. OME has been shown to co-occur with asthma, chronic sinusitis with nasal polyps, and atopy. The exudate in this type of OME has the form of thick, yellow, very sticky mucus and contains a large number of eosinophils. Patients with OME sometimes develop hearing loss in the high-pitch range, and there have been reports of sudden sensorineural hearing loss. OME is also common in patients with eosinophilic phenotypes of chronic sinusitis with nasal polyps. OME is most common in women after the age of 50 years [1, 6, 7].
Aim
The purpose of this study was to assess the co-occurrence of OME and allergic rhinitis and asthma to confirm their common inflammatory mechanisms. We also evaluated the potential risk of tonsillectomy in individuals with a history of OME and analyzed many factors that tend to protect against OME.
Material and methods
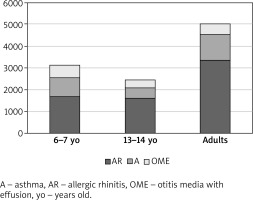
The study group consisted of 18,617 respondents, nearly 7.5% of whom had been diagnosed with OME (Table 1). A substantial proportion of the study population had been diagnosed with allergic rhinitis (stratified by age: 37.9% of 6–7-year-olds, 34.6% of 13–14-year-olds, and 36.0% of adults; stratified by place of residence: 37.7% of urban residents and 22.9% of rural residents) and asthma (stratified by age: 19.3% of 6–7-year-olds, 10.2% of 13–14-year-olds, and 12.4% of adults; stratified by place of residence: 14.0% of urban residents and 9.1% of rural residents).
Table 1
Study group characteristics
Our study was conducted with the use of European Community Respiratory Health Survey (ECRHS) II and an International Study of Asthma and Allergies in Childhood (ISAAC) questionnaires [8, 9] adopted for Central and Eastern Europe. The study was a part of the project titled Implementation of a System for the Prevention and Early Detection of Allergic Diseases in Poland. This project had been designed to include a population of adults (aged 20–44 years) and populations of children (aged 6–7 years) and adolescents (aged 13–14 years) – with the first age group meeting the ECRHS standard and the latter two meeting the ISAAC standard – who reside in eight of the most populated Polish urban areas (the cities of Gdansk, Wroclaw, Poznan, Katowice, Krakow, Lublin, Bialystok, and Warszawa) and one rural area (Krasnostawski County). The areas of residence had been selected in a non-random manner, whereas the respondents in the individual areas had been selected randomly (drawn). The draw had been designed to select a population that would be representative of the given population. The sampling frame was based on the Polish citizen ID (PESEL) number, with the associated information on the person’s name, age, sex, and address. The PESEL database is the State Systems Department at the Polish Ministry of the Interior and Administration. The data on the proportion of the individual stratification subgroups were obtained based on information from a Regional Data Bank. This study was conducted with a computer-assisted personal interviewing (CAPI) technique. Thirty percent of all ECRHS and ISSAC questionnaire respondents were supposed to undergo additional medical evaluations (skin-prick tests, lung function tests, and serum IgE levels) conducted in accordance with the established diagnostic standard for allergic conditions.
Statistical analysis
The study had been approved by the Ethics Committee of the Medical University of Warsaw (KB/206/2005) and the Inspector General for the Protection of Personal Data. This study made use of contingency tables and analyzed the prevalence of individual allergic conditions. Logistic regression, odds ratio (OR), and the 95% confidence interval (CI) for that odds ratio were used to determine the risk of allergic conditions potentially affecting otitis media. The level of statistical significance was set at α = 0.05.
Results
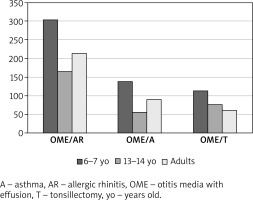
OME most commonly co-occurred with allergic rhinitis (303 cases among the 6–7-year-olds, 166 among the 13–14-year-olds, and 214 among adults) and with asthma (139 cases among the 6–7-year-olds, 57 among the 13–14-year-olds, and 91 among adults) (Table 1). Moreover, OME eventually led to adenoidectomy in a substantial proportion of respondents (114 of the 6–7-year-olds, 77 of the 13–14-year-olds, and 61 of adults) (Figures 1, 2).
Figure 1
The prevalence of otitis media with effusion (and other selected conditions) established as part of an epidemiological study
Our analysis indicated that OME increases the risk of allergic rhinitis by nearly two-fold. OME also considerably increased the risk of developing asthma (Figure 3). All respondents, irrespective of their place of residence (urban or rural area), had a comparable estimated risk of developing the evaluated conditions during the course of OME (OR = 1.73, 95% CI: 1.544–1.942 for urban residents with allergic rhinitis; OR = 1.752, 95% CI: 1.191–2.577 for rural residents with allergic rhinitis). Interestingly, OME increased the likelihood of adenoidectomy by more than two-fold, particularly in urban areas (OR = 2.402, 95% CI: 1.813–2.449). The most common positive reactions in the skin allergy tests of respondents diagnosed with OME were to perennial allergens, i.e. Dermatophagoides pteronyssinus, Dermatophagoides farinae, and cat allergens, with fewer positive reactions to grass, cereal, and birch allergens. Allergen application triggered a skin reaction significantly more commonly in residents of large cities than in rural residents. These were, however, mostly low-intensity localized skin reactions (in the form of a blister measuring 3–6 mm in diameter (Table 2)). The analyzed risk factors facilitating or preventing OME development include: the number of siblings (the more children in a family, the higher the risk of developing OME (OR = 2.00, 95% CI: 1.15–1.346), consumption of dairy products rich in lactic acid bacteria (OR = 1.27, 95% CI: 1.05–1.55 in 6–7-year-olds and OR = 1.26, 95% CI: 1.01–1.58 in adults (OR = 1.26, 95% CI: 1.09–2.49 in urban residents and OR = 1.65, 95% CI: 1.09–2.49 in rural residents). Smoking was also found to increase the risk of OME in adults (OR = 1.20, 95% CI: 1.00–1.44), particularly in urban area residents (OR = 1.28, 95% CI: 1.05–1.55). Preventive measures, such as antibiotic use three times before the age of 1 year, reduced the risk of OME development in the group of 6–7-year-olds (OR = 0.76, 95% CI: 0.62–0.92), 13–14-year-olds (OR = 0.78, 95% CI: 0.62–0.99) and 20–44-year-olds (OR = 0.70, 95% CI: 0.52–0.95), with the rural and urban subgroups showing comparable figures (OR = 0.74, 95% CI: 0.65–0.85 and OR = 0.50, 95% CI: 0.41–0.60, respectively) (Figure 4). Other parameters analyzed for their potential effect on OME development included: a history of measles, a history of chickenpox, or going to kindergarten at an early age did not show any significant protective or causative effects on OME.
Table 2
The absolute numbers (n) and proportions of positive skin allergy tests in the otitis-media-with-effusion group
Discussion
The concept of multimorbidity, i.e. concurrent existence of two or more chronic diseases, with the associated multidisciplinary patient care, has recently drawn much attention in the fields of allergy, otorhinolaryngology, and other medical specialties. In 2015, Bousqet et al. described multimorbidity involving the following allergic conditions: allergic rhinitis, asthma, and atopic dermatitis, and emphasized their similar immune and non-immune pathomechanisms [10]. Cingi et al. reported the concept of multimorbidity with respect to allergic rhinitis. Those authors proposed a classification of allergic rhinitis comorbidities into (a) allergic conditions (asthma, atopic dermatitis, food allergy, anaphylaxis), (b) conditions affecting the anatomical structures in proximity to the nose (otitis media, sinusitis, conjunctivitis), (c) problems with sleep and concentration, and (d) turbinate hypertrophy [11]. Our study was an attempt to evaluate the co-occurrence of OME and selected allergic conditions and analyse the effect of other factors on OME development. Out of the 18,617 respondents evaluated in our study 1,405 (including 766 [7.7%] females and 639 [7.4%] males) had been diagnosed with OME. OME was most common in the youngest age group evaluated (6–7-year-old children) with 567 (12.6%) cases, with a lower proportion of affected adolescents aged 13–14 years (369; 7.8%), and the lowest proportion of affected adults (469; 5%). These figures are consistent with other relevant literature reports. OME is the most common ear condition of childhood and the most common cause of hearing loss in children. As much as 80% of children up to 10 years of age have had an OME episode, with the peak incidence between the ages of 2 and 5 years of age. The proportion of those affected with OME decreases with their age, from 20% of the population of 2-year-olds to 8% of the population of 8-year-olds affected. The proportion of OME in the adult population is approximately 0.6% [1, 5, 12].
OME is a multifactorial disease, with the role of allergy in its pathogenesis already having been reported for many years. The mucous membrane of the middle ear and that of the upper and lower respiratory tract both derive from the ectoderm, which suggests that an allergic inflammation in the respiratory tract, may also affect the middle ear and involve the same inflammatory cells and mediators [5, 12]. This was demonstrated by Smirnova et al., who demonstrated that mediators of Th2-mediated inflammatory response, such as interleukins 4 and 5 (IL-4 and IL-5), play an important role in otitis media, especially in chronic otitis media [13]. Additionally, oedema of the mucous membrane in the nasal cavity and nasopharynx contributes to oedema of the pharyngeal orifice of the Eustachian tube resulting in Eustachian tube dysfunction, which, in turn, facilitates the development of OME [14].
Our study also demonstrated co-occurrence of OME and allergic rhinitis. Out of 567 children (6–7-year-olds), 369 adolescents (13–14-year-olds), and 469 adults with OME, allergic rhinitis was detected in 303 (53%), 166 (45%), 214 (45%), respectively. Our analysis indicated that OME increases the risk of allergic rhinitis by nearly two-fold. A 24-study meta-analysis by Zhang et al. evaluated the risk factors for otitis media. Those authors concluded that atopy and allergy increase the risk of ear inflammation [15]. Chantzi et al., who assessed 2,320 children, also confirmed a relationship between allergies and OME development [16]. A study by Norhafizah et al. involving 130 children with OME showed allergic rhinitis in 71 (80.3%) children with a chronic form of OME. Thus, that study demonstrated that allergic rhinitis is an important risk factor in the development of chronic OME (p < 0.0001). Interestingly, most of those children had chronic allergic rhinitis, and skin-prick tests showed the house dust mites Dermatophagoides pteronyssinus and Dermatophagoides farinae to be the most common causative allergens (86.2% and 87.7% of cases, respectively) [17]. A study by Mandel et al. showed positive skin-prick test results in 51 (41.8%) of the 122 evaluated patients with OME. The most common triggering allergens were house dust mites (22%), house pet (cat or dog) allergens (13.9%), moulds (13.1%), and grasses (10.7%) [18]. The skin-prick tests conducted in our study also showed allergies that predominantly involved perennial allergens, such as house dust mite (Dermatophagoides pteronyssinus and Dermatophagoides farinae) and cat allergens. Allergy to perennial allergens is associated with continual allergen exposure and chronic respiratory tract inflammation, which seems to increase the risk of developing OME.
Our study also demonstrated co-occurrence of OME and asthma in 24.5% of the evaluated 6–7-year-olds, 15.4% of the 13–14-year-olds, and 19.4% of the adults. OME was shown to increase the risk of developing asthma. The relationship between OME and asthma has been proven for a particular type of ear inflammation – eosinophilic OME. Optimal and successful treatment in asthmatics has been shown to help control the symptoms of eosinophilic otitis media. The two diseases have a common feature, which is active eosinophilic inflammation. The exudative fluid in OME contains elevated levels of the eosinophil chemotactic factors IL-5, eotaxin, and ecalectin [9]. Nguen et al. suggested that, according to the concept of united airway disease, allergic inflammation of the lower and upper airways also includes the middle ear. Conversely, another hypothesis states that due to the middle ear’s communication with the airways via the Eustachian tube, airway inflammation may be a risk factor for developing asthma, especially in children [19]. Kim et al. demonstrated a bi-directional relationship between otitis media and asthma. Their studies led them to a conclusion that asthma both increases the risk of otitis media and is a prognostic factor for the development of otitis media. The hazard ratio (HR) for otitis media was 1.46 in asthma patients (95% CI: 1.40–1.52; p < 0.001), whereas the HR for asthma was 1.43 in otitis media patients (95% CI: 1.36–1.50, p < 0.001) [20].
Adenoid enlargement within the first years of life is a morphological manifestation of the high immune activity resulting from exposure to allergens entering the nasopharynx via the oral and nasal routes. The enlargement of lymphatic tissue in response to infections, which are common in childhood, also increases the adenoid size. The role of adenoids in OME pathogenesis may be due to their compressing the pharyngeal orifice of the Eustachian tube, which results in mechanical and functional Eustachian tube dysfunction. Adenoid enlargement is a known risk factor for otitis media development, with OME constituting one of the indications for adenoidectomy [4, 21]. According to the results of our study, adenoidectomy had been performed in 114 (20.1%) of 6–7-year-old children, 77 (20.8%) of 13–14-year-old adolescents, and 61 (13%) of adults. Adenoidectomy was shown to have been two times more common among urban residents than rural residents (urban residents: OR = 2.402, 95% CI: 1.813–2.449). This is likely due to the fact that cities offer an easier access to otolaryngologists and have a higher number of facilities offering otolaryngologic services than rural areas.
Our study demonstrated that ‘non-medical’, i.e. demographic, social, and environmental, factors also play an important role in OME development. The greater number of people in the family, the higher the risk of OME (OR = 2.00, 95% CI: 1.15–1.346). Our observations are consistent with those reported by other authors. Norhafizah et al. demonstrated that 96% of the evaluated children with chronic OME came from large families of more than four family members per household and that having a sibling diagnosed with OME is an important risk factor (25% of children with OME had siblings who had also been diagnosed with this condition, whereas 11.9% of children with OME had a negative history of OME in their siblings) [17]. Another factor increasing the risk of OME in our study was cigarette smoking (adults: OR = 1.20, 95% CI: 1.00–1.44, particularly in urban areas: OR = 1.28. 95% CI: 1.05–155). Zernotti et al. also indicated that exposure to cigarette smoke and being raised in a large family are important risk factors for OME development [1]. Conversely, our study demonstrated that receiving antibiotics three times before the age of 1 year reduces the risk of developing OME in the future (the use of antibiotics three times before the age of 1 year reduces the risk of chronic otitis media in 6–7-year-old children (OR = 0.76. 95% CI: 0.62–0.92 in 6–7-year-olds, OR = 0.78, 95% CI: 0.62–0.99 in 13–14-year-olds, and OR = 0.70, 95% CI: 0.52–0.95 in 20–44-year-olds). This may be due to the fact that recurrent infections and bacterial colonization of the nasopharynx are important risk factors for OME development. Bacteria enter the middle ear through the Eustachian tube, whose anatomical configuration at this age facilitates bacterial entry [21].
Conclusions
Our study confirmed the complexity and multifactorial nature of OME aetiology and the important role of allergy in OME development. We also addressed the phenomenon of multimorbidity, which due to co-existence of various conditions, calls for a comprehensive multidisciplinary approach to diagnosis and treatment.