Introduction
Wound healing is a complex process which includes many immunological and physiological events [1–4]. Malnutrition is recognized as a major risk factor for wound chronicity [5]. Studies with animal models and clinical trials provided evidence that wound healing demands an optimal state of nutrition, and chronic energy and protein deficiencies markedly suppress the process [1, 2]. Even short-term malnutrition doubles the time of collagen synthesis. Collagen deficiency impairs epithelialization and granulation as well as the remodelling process [2, 6, 7]. On the other hand, increased nutrient requirements during the healing process, in particular of chronic wounds, can increase metabolic rates, leading to uncontrolled muscle breakdown and severe malnutrition [6]. In a randomized study carried out previously in our centre, we observed that malnutrition and the risk of malnutrition are common among patients with venous ulcers, particularly the elderly. Individuals with ulcers had significantly lower scores of nutritional state, lower body mass index (BMI) values, and lower values of arm and calf circumference when compared with patients without ulcers [8, 9]. According to the European Wound Management Association, the complex care of a patient with a wound should include nutritional screening and, if needed, optimal nutritional support [10].
It is estimated that nutritional support for individuals with wounds should provide an additional daily caloric intake of 30–35 kcal/kg body weight. The extra calories are needed to compensate for the increased anabolism during wound healing. Protein enables proper collagen synthesis, immune function, and fibroblast proliferation, and is essential for all stages of wound healing. In individuals with chronic wounds, a protein provision of up to 1.5 g/kg body weight is recommended [4]. Arginine plays an important role in wound healing, not only as a precursor of nitric acid, which regulates inflammation, but also as a substrate for protein synthesis [11, 12]. Among vitamins, A and C are particularly important for wound healing [1]. Vitamin A modulates the immune response during inflammation, exerting anti-inflammatory effects [13], and stimulates fibroblast proliferation [14]. Vitamin C is a cofactor in the process of collagen synthesis and regulates the immune response. From the minerals, zinc, selenium, and iron are beneficial for wound healing as they regulate the immune response, stimulate collagen production, and promote tissue repair and growth [2].
Chronic wounds often affect older or critically ill individuals, who may not be able to fulfil the increased nutritional demands with a regular diet [15]. Therefore, to ensure an optimal nutritional status in these patients, oral nutritional supplementation (ONS) is recommended [10, 16, 17]. Most studies exploring the efficacy of high-protein ONS focused on pressure ulcers. Protein-rich oral formulae containing arginine, zinc, and antioxidants were demonstrated to significantly improve the healing process [18–20].
The role of dietary supplementation in venous ulcer healing is less well documented. Active leg ulcers affect up to 0.6% of the general population [21, 22], and 70–80% of cases are of venous aetiology [21, 23]. Venous leg ulcers (VLU) develop because of chronic venous insufficiency. The current management strategy for venous ulcers is based mainly on compression therapy [24, 25], local treatment (debridement and dressings, limb elevation, pain relief, ankle exercises) and improved nutrition [10, 17, 24, 26–28].
Dressings used in the local treatment of VLU should be compatible with compression therapy and effectively absorb exudate under pressure. To minimize the possibility of infection in the VLU, topical treatment must also provide microbiological control (e.g., silver ion dressings and antiseptics).
Aim
The aim of this preliminary study was to investigate the impact of a 12-week specialized treatment program including supplementation with an energy-dense, high-protein Cubitan formula on the dynamics of venous wound healing.
Material and methods
This prospective, 12-week study included 35 adult (> 18 years) individuals with chronic leg ulcers of venous aetiology. The recruitment took place between 2017 and 2019. One patient withdrew after the control visit in week 2 of the study, hence 34 patients were included in the final analysis. The enrolled patients had wounds with areas between 5 cm2 and 50 cm2, with ankle-brachial index values of 0.9–1.3, lasting longer than 6 weeks. Exclusion criteria included the presence of wounds of mixed or undiagnosed aetiology, any type of artificial nutrition (e.g., feeding tubes), a state of nutrition score > 3 (according to the Nutritional Risk Screening 2002; NRS-2002) [29], a haemoglobin level < 10 g/dl, and the presence of coexisting chronic diseases or severe or chronic organ failure. In particular, the conditions that excluded patients from the study were uncontrolled diabetes mellitus (glycated haemoglobin level > 7%), advanced renal (serum creatinine concentration > 124 mmol (> 1.4 mg/ml)) or hepatic insufficiency (viral hepatitis B or C infection), moderate to advanced cardiac failure, chronic obstructive pulmonary disease or peripheral artery disease, current or previous (< 1 year since the last chemotherapy or radiotherapy treatment) neoplastic disease, current immunosuppressive therapy, connective tissue disease, sepsis, and osteomyelitis.
Data collection
Wound bacterial cultures, body mass index (BMI), ankle-brachial index and blood count measurements were performed in all patients at study entrance. All enrolled patients were treated according to current guidelines for wound care [11, 16, 17, 24, 30] and compression therapy (class 2) with short-stretch bandages. The type of dressing and frequency of its change were adjusted to the clinical state of the wound. In the inflammatory phase, absorbent dressings were applied, with or without silver ions, depending on the microbiological condition of the wound (e.g., AQUACEL® Foam, AQUACEL® Ag Foam, ConvaTec). In the phases of granulation and epithelialization, the new tissues were protected with hydrocolloid dressings (e.g., Granuflex® or Granuflex Extra Thin®, ConvaTec) or mesh contact layers. Each patient attended an outpatient clinic twice a week. In case of infection and local symptoms of infection, antibiotic susceptibility testing was performed, and appropriate targeted antibiotic therapy was administered accordingly.
During the study, each patient received 3 bottles per day (3 × 200 ml) of an energy-dense, protein-rich oral Cubitan (Nutricia) formula to be consumed throughout the day between meals. Such supplementation provided the patient with 128 kcal, 10 g of protein, and 1.5 g of L-arginine per 100 ml of formula, macro- and micronutrients (e.g., Na, K, Cl, Ca, Mg, Fe, Zn, Se), and vitamins (e.g., A, D, E, K, B6, B12, C). Treatment compliance was determined based on the number of returned bottle caps (Table 1).
Table 1
Compliance to oral nutritional supplementation throughout the study
| ONS consumption rate | 1st month | 2nd month | 3rd month | Whole study |
|---|---|---|---|---|
| ONS intolerance† | 0 | 0 | 0 | 0 |
| 20–59% weekly dosex | 3 | 1 | 1 | 1 |
| 60–74% weekly dosexx | 2 | 3 | 1 | 3 |
| 75–100% weekly dose | 29 | 30 | 32 | 30 |
The dynamics of wound healing were assessed by planimetry and photographic documentation every two weeks during the 12-week study. The primary endpoint, complete wound healing, was defined as a reduction in the wound area by 100%. The secondary endpoints included the change in the ulcer area (in cm2 and as a percentage change from baseline), the change in the ulcer area assessed for small (< 20 cm2) and large (≥ 20 cm2) ulcers (in cm2 and as a percentage change from baseline), and the percentage change (from baseline) in wound bed tissue composition, including epithelial tissue, granulation tissue, fibrin, and sloughy tissue.
The following clinical parameters were also assessed: serum prealbumin levels (at week 0, 6 and 12), pain intensity on a 1 to 10 scale (a maximum value from the current day and an average weekly value). Additionally, a WHOQOL-BREF questionnaire [31] was used for quality of life assessment at week 0 and 12 of the study.
The study protocol was approved by the local bioethical committee (No. KB 467/2016). All enrolled patients gave written informed consent to participate in the study. All experiments were performed in accordance with relevant guidelines and regulations.
Results
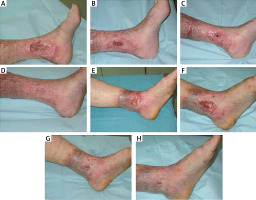
A summary of the baseline characteristics of the patients and their clinical condition is shown in Table 2. Photographs illustrating the healing process are included in Figure 1.
Table 2
Baseline characteristics of the study group (n = 35)
| Parameter | Mean (SD) | Median | Range | 95% CI |
|---|---|---|---|---|
| Age [years] | 71.7 (11.3) | 73.0 | 47.0–96.0 | 67.8–75.6 |
| Sex, male† | 13 (37%) | |||
| Body weight | 85.4 (17.2) | 85.0 | 55.0–135.0 | 79.5–91.3 |
| Height [m] | 1.7 (0.1) | 1.7 | 1.5–1.9 | 1.7–1.7 |
| BMI [kg/m2] | 30.2 (6.5) | 30.7 | 20.7–52.7 | 28.0–32.4 |
| BMI category [kg/m2]:† | ||||
| < 18.5 (underweight) | 1 (2.9%) | |||
| 18.5–24.99 (normal weight) | 9 (25.7%) | |||
| 25.0–29.99 (overweight) | 6 (17.1%) | |||
| 30.0–39.99 (obesity) | 13 (37.1%) | |||
| ≥ 40.0 (morbid obesity) | 6 (17.1%) | |||
| Serum prealbumin level [mg/dl] | 19.9 (5.6) | 19.0 | 11.0–33.0 | 17.9–21.8 |
| Compression therapy used†: | ||||
| None | 9 (25.7%) | |||
| Bandages | 1 (2.9%) | |||
| Kings | 25 (71.4%) | |||
| Ulcer duration [months] | 78.8 (101.7) | 36.0 | 2.0–420.0 | 43.8–113.7 |
| Comorbidities†: | ||||
| Osteoarthritis | 13 (37.1%) | |||
| Heart diseases | 9 (25.7%) | |||
| Rheumatoid arthritis | 7 (20.0%) | |||
| Diabetes mellitus | 6 (17.1%) | |||
| Atherosclerosis | 5 (14.3%) | |||
| Others | 18 (51.4%) | |||
| Ulcer characteristics†: | ||||
| Shallow | 30 (86%) | |||
| Deep: 1st, 2nd, 3rd degree | 8 (23%), 21 (60%), 4 (11%) | |||
| Single | 11 (31%) | |||
| Multiple | 24 (69%) |
Figure 1
Photographic documentation of venous leg ulcers healing. A–D – patient 1 in week 1, 3, 7, and 9, respectively. E–H – patient 2 in week 1, 3, 5, and 7, respectively
Most patients adhered well to recommendations regarding ONS intake, with consumption exceeding 75% of the suggested dose declared by 30 out of 34 patients (88%). Compliance to ONS remained stable throughout the study. Additionally, recorded adverse events were infrequent and transient. In the first week of the treatment, only 3 patients reported signs of ONS intolerance (e.g., diarrhoea, feeling of satiety, decreased appetite, nausea). For these patients, the following suggestions were made: decrease the volume of consumed formula, drink the formula in small portions, and gradually increase daily doses. This led to improved tolerance in the following weeks and thus to a greater number of returned caps in the 2nd and 3rd month of the study (Table 1).
Ulcer area changes
Complete wound healing was achieved by 6 patients (18%; p = 0.033) – all from the small ulcer subgroup (6/16, 37.5%; p = 0.32). When ulcer multiplicity was considered, 3 patients with single ulcers (3/10, 30%; p = 0.21) and 3 patients with multiple ulcers (3/24, 13%; p = 0.001) experienced complete wound healing. For patients who achieved complete wound healing, the median ulcer duration before study enrolment was 14 months (range: 6–60 months).
During the study, the median ulcer area decreased from 26.5 cm2 to 14.8 cm2. Statistically significant differences in the ulcer area from baseline were observed from week 2 to the end of the study. The percentage change in the initial ulcer area increased from 7.4% in week 2 to 43.6% in week 12, reaching statistical significance in week 8. A gradual decrease in ulcer size was also observed in subgroup analyses, including ulcer size and ulcer multiplicity (single vs. multiple ulcers), with significant differences from baseline observed from week 8 of the study. Detailed characteristics of changes in the ulcer area are given in Table 3 A.
Table 3
Changes in the ulcer area (part A), wound bed composition (part B) and pain intensity (part C) during a 12-week supplementation with Cubitan formula
| Part A | Ulcer area [cm2] | Percentage change from W0 | ||||
|---|---|---|---|---|---|---|
| Week | Median | Range | P (vs. W0)* | Median | Range | P (vs. W2)* |
| Overall analysis (n = 35†): | ||||||
| W0 | 26.5 | 3.3–56.5 | – | – | – | – |
| W2 | 26.1 | 0.0–60.0 | < 0.05 | 7.4 | –32.3–100.0 | – |
| W4 | 20.9 | 0.0–48.0 | < 0.05 | 17.2 | –22.2–100.0 | > 0.05 |
| W6 | 19.3 | 0.0–48.5 | < 0.05 | 19.4 | –95.8–100.0 | > 0.05 |
| W8 | 15.2 | 0.0–45.0 | < 0.05 | 26.9 | –84.2–100.0 | < 0.05 |
| W10 | 14.7 | 0.0–40.5 | < 0.05 | 34.2 | –55.8–100.0 | < 0.05 |
| W12 | 14.8 | 0.0–39.2 | < 0.05 | 43.6 | –116.8–100.0 | < 0.05 |
| Subgroup analysis: ulcer multiplicity: | ||||||
| Single ulcers (n = 11†): | ||||||
| W0 | 5.2 | 3.3–47.5 | – | – | – | – |
| W2 | 4.6 | 3.7–38.0 | > 0.05 | 14.3 | –32.3–50.0 | – |
| W4 | 3.9 | 2.1–40.1 | > 0.05 | 24.5 | 11.5–65.4 | > 0.05 |
| W6 | 3.8 | 2.1–40.8 | > 0.05 | 29.1 | 14.1–73.1 | > 0.05 |
| W8 | 3.0 | 1.3–39.6 | < 0.05 | 37.1 | 16.0–83.3 | > 0.05 |
| W10 | 2.6 | 0.3–38.4 | < 0.05 | 51.5 | 19.2–96.2 | < 0.05 |
| W12 | 2.0 | 0.0–37.9 | < 0.05 | 66.2 | 20.2–100.0 | < 0.05 |
| Multiple ulcers (n = 24): | ||||||
| W0 | 38.3 | 4.8–56.5 | – | – | – | – |
| W2 | 33.7 | 0.0–60.0 | > 0.05 | 4.0 | –12.4–100.0 | - |
| W4 | 28.7 | 0.0–48.0 | > 0.05 | 14.5 | –22.2–100.0 | > 0.05 |
| W6 | 31.9 | 0.0–48.5 | > 0.05 | 17.9 | –95.8–100.0 | > 0.05 |
| W8 | 29.6 | 0.0–45.0 | < 0.05 | 20.8 | –84.2–100.0 | < 0.05 |
| W10 | 27.0 | 0.0–40.5 | < 0.05 | 27.6 | –55.8–100.0 | < 0.05 |
| W12 | 25.5 | 0.0–39.2 | < 0.05 | 35.1 | –116.8–100.0 | < 0.05 |
| Subgroup analysis: ulcer size: | ||||||
| Small (< 20 cm2) ulcers (n = 16): | ||||||
| W0 | 5.4 | 3.3–17.5 | – | – | – | – |
| W2 | 4.9 | 0.0–19.1 | > 0.05 | 10.4 | –32.3–100.0 | – |
| W4 | 4.1 | 0.0–18.5 | > 0.05 | 26.7 | –22.2–100.0 | > 0.05 |
| W6 | 3.8 | 0.0–16.9 | > 0.05 | 30.8 | –95.8–100.0 | > 0.05 |
| W8 | 3.0 | 0.0–13.5 | < 0.05 | 37.1 | –84.2–100.0 | > 0.05 |
| W10 | 2.6 | 0.0–12.3 | < 0.05 | 49.5 | –55.8–100.0 | < 0.05 |
| W12 | 2.0 | 0.0–14.6 | < 0.05 | 63.4 | –116.8–100.0 | < 0.05 |
| Large (≥ 20 cm2) ulcers (n = 19†): | ||||||
| W0 | 47.5 | 20.9–56.5 | – | – | – | – |
| W2 | 41.4 | 18.8–60.0 | > 0.05 | 4.0 | –12.1–36.5 | – |
| W4 | 36.3 | 18.5–48.0 | > 0.05 | 14.6 | –11.2–51.6 | > 0.05 |
| W6 | 37.4 | 17.2–48.5 | > 0.05 | 17.8 | –31.7–55.4 | > 0.05 |
| W8 | 35.5 | 15.0–45.0 | < 0.05 | 20.4 | –21.4–68.8 | < 0.05 |
| W10 | 33.3 | 10.3–40.5 | < 0.05 | 27.9 | –40.1–64.6 | < 0.05 |
| W12 | 30.7 | 6.8–39.2 | < 0.05 | 36.5 | –36.8–68.8 | < 0.05 |
| Part B | Percentage tissue content | Percentage change from W0 | ||||
| Week | Median | Range | P (vs. W0)* | Median | Range | P (vs. W2)** |
| Epithelial tissue: | ||||||
| W0 | 10.0 | 0–25 | – | – | – | – |
| W2 | 15.0 | 0–100 | > 0.05 | 40.0 | 0–900 | - |
| W4 | 25.0 | 5–100 | < 0.05 | 133.3 | 0–1 200 | > 0.05 |
| W6 | 35.0 | 5–100 | < 0.05 | 200.0 | 0–1 600 | < 0.05 |
| W8 | 40.0 | 10–100 | < 0.05 | 216.7 | 0–1 700 | < 0.05 |
| W10 | 47.5 | 10–100 | < 0.05 | 308.3 | 33–1 900 | < 0.05 |
| W12 | 57.5 | 10–100 | < 0.05 | 375.0 | 66–1 900 | < 0.05 |
| Granulation tissue: | ||||||
| W0 | 15.0 | 5–85 | – | – | – | – |
| W2 | 40.0 | 0–80 | < 0.05 | 120.0 | –100–900 | – |
| W4 | 42.5 | 0–60 | < 0.05 | 145.0 | –100–1 000 | > 0.05 |
| W6 | 40.0 | 0–65 | < 0.05 | 100.0 | –100–1 100 | > 0.05 |
| W8 | 40.0 | 0–65 | < 0.05 | 106.7 | –100–1 200 | > 0.05 |
| W10 | 37.5 | 0–80 | < 0.05 | 90.0 | –100–1 300 | > 0.05 |
| W12 | 30.0 | 0–65 | > 0.05 | 73.3 | –100–900 | > 0.05 |
| Sloughy tissue, fibrin: | ||||||
| W0 | 70.0 | 5–95 | – | – | – | – |
| W2 | 40.0 | 0–85 | > 0.05 | 50.0 | –14.3–100.0 | – |
| W4 | 25.0 | 0–80 | < 0.05 | 65.7 | –7.1–100.0 | > 0.05 |
| W6 | 15.0 | 0–75 | < 0.05 | 77.4 | 0.0–100.0 | < 0.05 |
| W8 | 10.0 | 0–85 | < 0.05 | 83.3 | 5.6–100.0 | < 0.05 |
| W10 | 7.5 | 0–65 | < 0.05 | 88.9 | 27.8–100.0 | < 0.05 |
| W12 | 5.0 | 0–70 | < 0.05 | 94.4 | 22.2–100.0 | < 0.05 |
| Part C | Pain – daily maximum‡ | Pain – weekly average§ | ||||
| Week | Median | Range | P (vs. W0)* | Median | Range | P (vs. W2)* |
| W0 | 5.0 | 1.0–10.0 | – | 5.0 | 1.0–10.0 | – |
| W1 | 4.5 | 1.0–10.0 | > 0.05 | 4.0 | 1.0–10.0 | > 0.05 |
| W2 | 4.0 | 0.0–9.0 | > 0.05 | 4.0 | 0.0–9.0 | > 0.05 |
| W3 | 3.0 | 0.0–9.0 | > 0.05 | 3.0 | 0.0–9.0 | > 0.05 |
| W4 | 3.0 | 0.0–8.0 | > 0.05 | 3.0 | 0.0–8.0 | > 0.05 |
| W5 | 3.0 | 0.0–8.0 | < 0.05 | 3.0 | 0.0–8.0 | > 0.05 |
| W6 | 3.0 | 0.0–8.0 | < 0.05 | 3.0 | 0.0–8.0 | < 0.05 |
| W7 | 2.5 | 0.0–6.0 | < 0.05 | 3.0 | 0.0–7.0 | < 0.05 |
| W8 | 2.0 | 0.0–6.0 | < 0.05 | 2.0 | 0.0–7.0 | < 0.05 |
| W9 | 2.5 | 0.0–6.0 | < 0.05 | 2.0 | 0.0–6.0 | < 0.05 |
| W10 | 2.5 | 0.0–6.0 | < 0.05 | 2.0 | 0.0–6.0 | < 0.05 |
| W11 | 2.0 | 0.0–6.0 | < 0.05 | 2.0 | 0.0–6.0 | < 0.05 |
| W12 | 2.0 | 0.0–6.0 | < 0.05 | 2.0 | 0.0–6.0 | < 0.05 |
* Calculated with the Friedman test (p = 0.0001 for both absolute change and percentage change analyses) and post-hoc Dunn’s test. P-values < 0.05 are marked in bold.
** Calculated with the Friedman test (p = 0.0001, 0.025, 0.0001 for analyses of epithelial tissue, granulation tissue and sloughy tissue, fibrin, respectively) and post-hoc Dunn’s test. P-values < 0.05 are marked in bold.
We monitored changes in the composition of the wound bed to gain a better insight into the dynamics of the healing process (Table 3 B). The median content of epithelial and granulation tissue increased systematically. On the other hand, the fibrin content in the wound bed decreased.
Prealbumin levels
Changes in prealbumin levels throughout the study were not statistically significant. However, during the first 6 weeks of the study, the mean prealbumin levels increased noticeably from 19.9 mg/dl to 21.6 mg/dl, and remained stable during the last 6 weeks of the study (Table 4).
Changes in chronic venous disorders, pain intensity and quality of life
A significant decrease in the CEAP-C6 classification [31] score was observed during the study, with median values of 12 (range: 9–16) in week 0, and 10 (range: 0–15) in week 12 (W0 vs. W12; p = 0.0004).
Both patient self-assessments for pain intensity, the maximum value from the day and the average value from the week, constantly decreased from 5 to 2 throughout the study (Table 3 C). When compared to the baseline assessments for week 0, a decrease in pain intensity was statistically significant starting from week 6 for the daily assessment and week 7 for the weekly assessment.
The results of the quality of life assessment using the WHOQOL-BREF questionnaire are presented in Table 5. Significant improvements between assessments at study entrance (W0) and at the end of the study (W12) occurred on the general and environment scales. No major changes were observed in the physical, psychological, and social domains.
Table 5
Life quality assessment in WHOQOL-BREF questionnaire (Skevington, Lotfy, O’Connell, & WHOQOL Group, 2004) for patients with venous leg ulcers supplemented with Cubitan formula (n = 35†)
| WHOQOL-BREF scale points | Initial assessment (W0) | End of study assessment (W12) | P-value* | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Physical | 22 | 17–29 | 23 | 17–27 | 0.796 |
| Psychological | 20 | 14–25 | 20 | 15–26 | 0.0792 |
| Social | 11 | 8–15 | 11 | 8–15 | 0.3942 |
| Environment | 27 | 19–40 | 29 | 19–40 | 0.0003 |
| General | 80 | 62–107 | 83 | 65–104 | 0.0013 |
Discussion
As a result of increasing life expectancy and population ageing, VLU is becoming a significant economic and epidemiological public health issue. Its prevalence increases with age, affecting as much as 2% of individuals older than 80 years [32–36]. VLU are caused by venous insufficiency, calf muscle pump failure, and vascular valve incompetence that results in venous hypertension [37]. Therefore, the origin of VLU is distinct from that of pressure [38], neuropathic, or arterial ulcers [37]. VLU are of chronic course, with high recurrence rates of up to 28% per year [39]. Additionally, they are painful, prone to infection, and may restrict the patient’s mobility, and thus drastically deteriorate the quality of life [40, 41]. VLU are associated with severe psychosocial consequences, such as depression and disturbances in sleep and daily living activities [41]. The management of VLU is complex and requires multi-disciplinary care. It involves compression therapy, which reduces venous hypertension by applying external pressure, the use of specialized dressings, pharmacotherapy, and surgical treatment [10, 16]. Additionally, the current VLU management guidelines recommend an assessment of the nutritional state of the patients and adequate application of nutritional support to promote wound healing [10, 16, 17, 24].
Malnutrition affects 5–10% of older individuals living independently, and up to 65% and 85% of those in hospitalized and home nursing populations, respectively (encompasses undernutrition (resulting from insufficient or inadequate food consumption), overnutrition, and deficits in particular nutrients) [14, 42]. A pilot study including nine hospitalized patients with leg ulcers, including VLU, showed that the nutritional status of individuals with chronic wounds is inadequate, and oral food intake is insufficient to cover their energy and protein requirements [15]. Patients with VLU were shown to have protein, vitamin A and E, and zinc deficiencies [43, 44]. Moreover, the intake of proteins, vitamins, and minerals among elderly patients, who are at a high risk of VLU, is insufficient [45, 46]. On the other hand, the demand for both energy and building substrates greatly increases during the wound healing process [2]. The shortage of endogenous substrates in malnourished patients impairs and delays the healing process by prolonging the inflammatory phase, restricting collagen synthesis, and impeding the immune function [47]. Using ONS, which provides an additional, easy-to-consume source of energy, proteins, essential micronutrients, amino acids, and vitamins, may be therefore beneficial in VLU patients.
Here, we showed that professional wound management in a specialized medical centre, together with energy-dense high-protein oral supplementation, led to a significant clinical improvement in patients suffering from VLU. Moreover, we provided evidence that a relatively short, 3-month treatment allowed for complete wound healing in 6 patients with long-term (≥ 1 year) ulcers (4/6) and very long-term (≥ 3 years) ulcers (2/6). This underlines the high efficacy of such a complex approach that combined professional care in a specialized centre, the use of advanced dressings, and nutritional support with energy-dense high-protein ONS enriched with arginine, zinc, and vitamins.
Throughout the study, the dynamics of healing was unequivocal. In 12 weeks, the median ulcer area decreased from 26.5 cm2 to 16.8 cm2, and the decrease was the most pronounced in the first 6–8 weeks of the study. The same observation was made for the subgroup of patients with small and single ulcers. Interestingly, changes in prealbumin levels throughout the study followed a similar pattern: the highest increase occurred in the first 6 weeks of the study and the levels remained stable in the next 6 weeks. The baseline median serum prealbumin was at the lower limit of the normal range, revealing the presence of protein depletion in the study group and suggesting that malnutrition was common among the patients despite a high BMI. Therefore, supplementation with the high-protein Cubitan (Nutricia, Hoofddorp, the Netherlands) formula may have corrected the nutritional deficit, and thus contribute to the enhanced healing rates observed in the first part of the study. In the group of patients with large and multiple ulcers, the healing progress remained stable during the 12 weeks of the study. This observation agrees with clinical experience that more complicated, larger, and multiple ulcers need longer time to heal [48–50].
The dynamics of the wound healing process throughout the study were observed in the changes in wound bed composition, typical of the healing process. The initial increase in the granulation tissue content, together with the gradual increase in epithelial tissue content, and the associated decrease in sloughy tissue and fibrin indicated progress in the healing process.
Along with the progress in wound healing, we observed an improvement in the patients’ condition and well-being. A significant decrease in pain intensity was achieved in week 6 and was in line with the progress in the wound healing process. The quality of life increased markedly at the end of the study when compared to baseline. This confirmed the earlier observations that VLU may negatively impact the patients’ well-being and a complex treatment may reverse these effects.
Literature focused on the analysis of oral supplementation in VLU healing is scarce. A recent systematic review describes only three papers that included dietary intervention for VLU management [51]. Two of these studies described the role of a single dietary component supplementation of vitamin D [52] and folic acid [53] on the course of VLU healing. Both interventions improved the healing process when compared to standard treatment, but the effect of vitamin D supplementation was not statistically significant. Another study proved the efficacy of a complex, flavonoid-rich nutraceutical product in VLU management, which contributed to enhanced healing and reduced recurrence rates [54]. There is no clear evidence, however, for the possible impact of an energy-dense protein-rich ONS on VLU healing. Raffoul et al. evaluated the impact of a high-calorie high-protein ONS on the healing process of 9 patients with various leg ulcers, including 3 cases of VLUs and pressure and ischemic ulcers. Complete wound healing was achieved in all cases [15]. A complex, energy-dense ONS with high protein content, enriched with vitamin C and zinc, was applied in six individuals with VLU [55]. At the end of the 9-month intervention, progressive wound healing was observed in most patients. However, because these studies used very small sample sizes, strong conclusions on the role of nutritional support in VLU treatment cannot be drawn. In contrast, the benefits of complex ONS are much better documented in the management of pressure ulcers. Cereda et al. showed that malnourished patients receiving an energy-dense, protein-rich ONS enriched with arginine, zinc, and vitamins, had improved healing parameters compared with those receiving a standard hospital diet or a high-calorie and protein ONS [18, 19]. Similarly, Desneves et al. demonstrated the benefits of an ONS enriched with arginine, zinc, and vitamin C on pressure ulcer healing compared to an ONS with high calorie and protein content only [56]. This indicates the key role for these nutrients in promoting wound healing. The high efficacy of so-called wound-ONS in ulcer healing, including arginine, zinc, and vitamin C, has also been described in original research papers [18–20] and review papers [57, 58]. In our study, we used an ONS formula containing arginine, zinc, and vitamins, which has been demonstrated to be highly efficacious.
Overweight and obesity are recognized as independent risk factors for VLU development [59, 60], and excess body mass is associated with delayed VLU healing [48]. In the above-mentioned studies, patients with normal or low body weight prevailed [15, 52–55]. By contrast, most patients recruited in this study had a BMI > 30 kg/m2. We observed that patients with a high BMI were still characterized by low prealbumin levels, which reached values even below the normal range in some cases. Because excess intake of food is the reason behind many cases of obesity, individuals with high BMI are generally not recognized as malnourished. However, numerous studies demonstrated the opposite. Morbidly obese individuals have been shown to have mineral (e.g., selenium, zinc, iron) and vitamin (e.g., B1, B12, D) deficits [61]. Recent studies assessing the nutritional status of obese patients prior to bariatric surgery revealed that, despite an excess of calories, the intake of micronutrients was inadequate, leading to micronutrient deficiencies [62–64]. Therefore, due to the high prevalence of excess body weight in individuals with VLUs, nutritional supplementation appears to be particularly crucial to ensure optimal conditions for the wound healing process.
An individualized selection of adequate wound dressings according to the phase of healing can markedly improve the healing process. Here, we used advanced moisture-retentive AQUACEL® Foam dressings for effective exudate management, AQUACEL® Ag Foam for infection and inflammation control, and, in the later phases of healing, hydrocolloid dressings- Granuflex® or Granuflex® Extra Thin to support sensitive newly produced tissues. AQUACEL® Foam dressings are characterized not only by their excellent absorbent and exudate handling capacities, but also for the comfort they provide to patients in terms of painless application and removal [65]. Foam dressings were also shown to reduce the activity of matrix metalloproteinases, MMP-2 and MMP-9 [66]. Such modulation may exert an additional favourable effect on the healing process as increased levels of metalloproteinases lead to excessive tissue degradation and can contribute to delayed wound healing [67].
In our study, adherence to the recommended ONS was satisfactory, demonstrating that the formula was well accepted by the patients. The rate of patient compliance to scheduled ONS intake reached 90%, which is similar to the 84.8% reached in a corresponding study focused on pressure ulcers [18]. Similar percentages were described in a systematic review by Hubbard et al., where compliance with ONS was 78% overall and 81% for individuals in community settings specifically [68].
This study has certain limitations. The results are promising but still preliminary as no placebo group was included in the analysis. Additionally, the study group was heterogeneous in terms of patient body weight. It included patients with normal BMI but also overweight and obese individuals, who are at a high risk of chronic venous insufficiency and VLU.
Conclusions
We provided evidence that a complex management of VLU in a specialized centre including the application of advanced dressings and an energy-dense high-protein ONS enriched with amino acids, vitamins, and minerals, might benefit the healing process. The high acceptance levels of the ONS among older individuals indicate that it can serve as an easy-to-consume source of nutrients, allowing the patients to reach the nutritional status optimal for the wound healing. Such status is especially difficult to achieve with a standard diet in elderly patients, who often experience a decrease in appetite.








