Introduction
The increasingly frequent use of computed tomography (CT)-based imaging in clinical practice has led to the detection of increasingly high numbers of lung nodules (LNs) [1]. Accordingly, rates of video-assisted thoracoscopic surgery (VATS) as a means of removing these LNs for analysis have also risen in recent years [2–4]. To improve the technical success rates of VATS sublobar resection procedures and to decrease rates of conversion from VATS to thoracotomy, preoperative LN localization has become an increasingly common strategy [5]. Widely utilized localization materials include hook-wire, micro-coil, and liquid-based materials [6, 7], all of which can achieve high (> 90%) success when used for LN localization [8, 9]. Compared to hook-wire-based localization, the use of liquid localization materials can yield better safety outcomes [9], while also reducing the duration of the localization procedure relative to that required for micro-coil localization [8].
Approximately 20% of LN patients have more than one moderate-to-high malignant risk LNs [10, 11]. Simultaneously resecting multiple LNs has the potential to lower the risk of tumor progression during the period between staging procedures while also shortening patient treatment time [12]. As such, the ability to successfully and simultaneously localize all target LNs is critical to the successful execution of one-stage VATS procedures in patients with multiple LNs [10, 11].
Many researchers have assessed the clinical effectiveness and safety of hook-wire and coil localization for patients with multiple LNs [4, 12]. Compared to single LN localization, simultaneous hook-wire or microcoil localizations for multiple LNs were prone to pneumothorax and hemorrhage [4, 12]. However, studies regarding liquid material localization for patients with multiple LNs are still lacking.
Aim
The present study was designed to evaluate the efficacy and safety of CT-guided indocyanine green (IG) localization in patients with multiple ipsilateral LNs, comparing the success of such localization to that in patients undergoing localization of a single LN.
Material and methods
This was a retrospective single-center study approved by the ethics committee of our hospital. Written informed consent was not required owing to the retrospective design of these analyses.
Study design
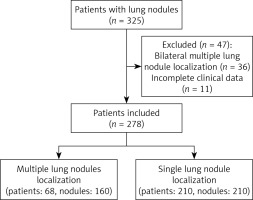
In total, 278 LN patients who underwent CT-guided IG localization and subsequent VATS resection between June and September 2022 were included in the present study, of whom 68 (24.5%) and 210 (75.5%) respectively underwent localization of multiple ipsilateral LNs and a single LN.
Indications for IG-based localization included: (a) solid LNs ≤ 10 mm in diameter; (b) solid LNs with a lesion-nearest pleura distance ≥ 10 mm; and (c) ground glass nodule (GGN) with no restrictions on diameter or lesion-pleura distance. Patients were excluded if (a) they underwent localization of multiple bilateral LNs or (b) they exhibited any severe comorbidities.
The indications of resection of LNs were: (a) recent increase in LN size; (b) recent occurrence or enlargement of solid components in the GGNs; and (c) suspicious lung cancers based on the Lung-RADS guideline [1].
CT-guided localization
IG localization was performed under local anesthesia using a 16-row CT (Siemens, Berlin, Germany) instrument. Procedures were performed by two interventional radiologists, each with over 5 years of experience. Patients were appropriately positioned based on the locations of the target LNs, after which a 21G needle (Argon Medical Devices, Inc, TX, USA) was used to puncture the lung parenchyma based on the direction of the target LN. CT scanning was performed repeatedly to ensure appropriate needle tip localization, and the tip was adjusted until it was within 1 cm of the target nodule. IG (2.5 mg/ml, 0.3 ml) was then gradually injected as the needle was slowly removed such that IG was visibly present on the visceral pleura. All LNs were localized with a one-stage CT-guided protocol.
VATS procedures
All VATS procedures were performed within 3 h following IG localization. Initially, IG fluorescence was used to guide sublobar VATS resection. When VATS visualization was sufficient to ensure adequate margins for the target LN, wedge resection was performed, whereas segmental resection was otherwise conducted. After being resected, tissue samples were sent to the Department of Pathology, where they underwent rapid pathological assessment. Pathological results were used to guide subsequent procedures, with no further resection being performed when the nodule was classified as cancer in situ or below, while systematic lymph node dissection (SLND) but not lobectomy was conducted in cases where an LN was classified as a mini-invasive cancer, and both lobectomy and SLND were performed when an LN was identified as an invasive cancer. In cases where multiple invasive lung cancers were identified, lobectomy was performed for the LN exhibiting the highest tumor staging.
Definitions
IG localization was deemed a technical success when IG fluorescence was visible on the surface of the lung without diffusion away from the site of injection [9]. Wedge/segmental resection procedures were considered a technical success when the target LN was fully present in the resected segment of tissue [9]. The duration of localization was the interval between the time at which patients initially lay down on the CT table to the time that IG injection was complete [13], while VATS time was the interval between the initial incision and wound closure [13]. Lung hemorrhage was defined based on the observation of new consolidative or ground glass opacity near the CT needle tract [14].
Statistical analysis
Quantitative data are given as means ± standard deviation and compared via independent t-tests, while categorical data are given as n (%) and compared via χ2 tests. Logistic regression analyses were used to detect risk factors associated with complications after localization, with variables that were significant (p < 0.1) in univariate analyses being included in a multivariate analysis. P < 0.05 was the significance threshold, and SPSS 16.0 (SPSS Inc., IL, USA) was used to conduct all analyses.
Results
Patients
In total, 160 LNs were localized in the 68 patients in the multiple localization group whereas a single LN was localized in each of the 210 patients in the single localization group. For details regarding the study flow (Figure 1). For details regarding the baseline characteristics of patients in these study groups (Tables I and II).
Table I
Comparison of patients’ baseline data between 2 groups
Table II
Comparison of lung nodules’ baseline data between 2 groups
IG localization
IG localization procedures were a technical success in 100% of cases in both patient groups (Table III). In total, 20 (29.4%) patients in the multiple localization group had to undergo positional adjustments during these procedures. The mean localization durations in the multiple and single localization groups were 11.3 ±4.7 min and 6.3 ±2.7 min, respectively (p = 0.001).
Table III
Localization and VATS related results
Pneumothorax affected 22 (32.3%) and 15 (7.1%) patients in multiple and single localization groups, respectively (p = 0.001, Table II), but none of these patients experienced symptoms, nor did they need to undergo chest tube insertion. In the multiple localization group, pneumothorax occurred after the first, second, and third LN localization procedures in 5, 16, and 1 patient, respectively. The risk factors found to be associated with multiple LN localization-related pneumothorax incidence included longer localization time (p = 0.014) and position change (p = 0.004) (Table IV).
Table IV
Predictors of pneumothorax for localization of multiple lung nodules
Lung hemorrhage affected 14 (20.6%) and 20 (9.5%) patients in multiple and single localization groups, respectively (p = 0.016, Table II), but none of these patients experienced hemorrhage-related symptoms. Longer localization time (p = 0.023) was identified as a risk factor associated with lung hemorrhage in patients undergoing localization of multiple LNs (Table V).
Table V
Predictors of lung hemorrhage for localization of multiple lung nodules
VATS resection outcomes
VATS resection procedures were successfully conducted within 3 h following localization in all cases, and these procedures were a technical success in 100% of patients in both groups (Table III). Conversion to thoracotomy was not required in any cases, and one-stage resection was successfully performed for all target LNs in the multiple LN group. Additional lobectomy was required for 11 and 38 patients due to the identification of invasive lung cancers, and the mean VATS durations in the multiple and single localization groups were 11.3 ±4.7 min and 6.3 ±2.7 min, respectively (p = 0.001). The mean respective blood loss in the multiple and single localization groups was 17.3 ±11.0 ml and 14.1 ±7.3 ml (p = 0.027). Final pathological diagnosis results for all LNs in this study are summarized in Table III, and rapid pathological results matched final pathological diagnoses in all cases.
Discussion
The present study was performed to explore the relative safety and efficacy of CT-guided IG localization of multiple ipsilateral LNs as compared to localization of a single LN. Technical success rates for this localization strategy and subsequent VATS sublobar resection procedures were 100% in all patients included in the present study. This demonstrates the feasibility of CT-guided simultaneous one-stage IG injection procedures for localization of multiple LNs and the ability to use such localization to guide one-stage VATS sublobar resection of these multiple LNs.
Approximately 1–8% of lung cancer patients have multiple lung cancers [15], and one-stage VATS resection can be beneficial for patients with multiple lung cancers [12]. In this study, 68 of 278 (24.5%) patients required multiple LNs localization, and this rate was comparable to those (22.2–24.1%) in previous studies regarding multiple LN localization [16, 17]. In theory, simultaneous resection procedures should be superior to two-stage procedures as they should be able to better lower the odds of disease progression [12].
Pneumothorax and lung hemorrhage were the most common complications after multiple LN localization with the incidences of 27.5–56.8% and 18.9–60% [16–18]. In this study, pneumothorax and lung hemorrhage rates in the multiple LN group were 32.3% and 20.6%, respectively, which were consistent with the previous studies. Furthermore, pneumothorax and lung hemorrhage rates were significantly higher in patients who underwent localization of multiple LNs relative to those who underwent localization of a single LN, in line with prior reports pertaining to CT-guided multiple LN localization [16, 17]. However, none of these complications were symptomatic and these complications did not require special treatment, such as chest tube insertion. Therefore, IG localization for multiple ipsilateral LNs is clinically safe.
Localizing multiple nodules necessitates additional needle pathways, patient positional changes, and prolonged localization procedures. Here, longer localization time was identified as a risk factor significantly associated with the odds of pneumothorax and lung hemorrhage, whereas positional change was specifically associated with the risk of pneumothorax. Position change has also been confirmed as a risk factor of pneumothorax in some previous studies [4, 16]. Therefore, we should choose the most reasonable puncture route, and avoid position change as far as possible. Longer localization time was confirmed in a previous study regarding methylene blue localization for multiple LNs [19]. Longer localization time may be caused by repeat puncture procedures, and the air could flow into the pleural cavity more easily. In the 22 patients who experienced pneumothorax in the multiple group, 17 (77.3%) patients had the pneumothorax after the second or third LN localization. This finding may also confirm that longer localization time was a risk factor of localization-related complications.
Just 11 (16.2%) of 68 multiple LN patients in this study were diagnosed with invasive adenocarcinoma and needed to undergo subsequent lobectomy, while multiple sublobar resection was required in the remaining 57 (83.8%) patients. Thus, preoperative IG localization can help patients with multiple LNs retain maximal lung function.
There are some limitations to this study. For one, this was a retrospective study and patients were grouped based on numbers of LNs such that randomization could not be performed. Accordingly, baseline data such as gender, age, LN diameter, and smoking history varied between these groups. Even so, these factors were not identified as risk factors associated with complication rates in multivariate analyses. Additionally, outcomes associated with IG localization were not compared to those associated with other localization materials in patients with multiple LNs. Hence, additional prospective clinical research will be essential to better compare different localization materials in order to define the optimal approach to simultaneously localizing multiple LNs.
Conclusions
CT-guided IG localization can be effectively and safely used to localize multiple ipsilateral LNs, although it requires a longer operative duration than localization of a single LN and is associated with a higher risk of pneumothorax and lung hemorrhage. Avoiding position change and reducing the localization time as much as possible may help to reduce the incidence of localization-related complications.