Introduction
The incidence of renal cell carcinoma (RCC) is the highest in Western population (2–3% of all cancer types). In kidney cancer, 80–90% of all cases are clear cell carcinomas. Cutaneous metastatic (CM) can be observed in nearly 25% of all RCC cases. At diagnosis, 16% of patients with RCC are found to have metastases and, therefore, present a poor prognosis (cancer specific survival (CSS) hazard ratio (HR) 33.23; 95% CI: 28.18–39.18; p < 0.001) [1, 2]. The most common locations for metastasis of RCC are the lungs (50%), bones (33%), lymph nodes (6–11%), liver (8%), and adrenal glands and brain (3%) [3]. Cutaneous metastasis of RCC is rare and usually related to a late symptom of the disease. These metastases develop within 36 months after the primary malignancy is initially diagnosed (1–177 months), but occasionally skin metastases are the manifestation of an undetected, asymptomatic RCC [4]. One meta-analysis reported the overall incidence of cutaneous metastases in all tumour types of 5.3% [5]. Other recent report suggests this prevalence to be from 1% to 4.3% [6].
The case presented here is of a human with scalp metastasis of clear RCC, found to be a primary manifestation of advanced cancer [7].
Aim
The study aimed to review the literature and present a case study of cutaneous metastasis in primary genitourinary malignancy, especially RCC in order to broaden the related knowledge.
Material and methods
In the first stage of our work, the review of the literature about cutaneous metastasis in primary genitourinary malignancy, especially renal cell carcinoma was performed. Pubmed, Science Direct and Web of Science were used for the analyses.
The work was performed according to the guidelines of the Declaration of Helsinki. Because this is a case retrospective study, the Opinion of the Bioethical Committee was not required. Considering that CM in RCC is relatively rare (the first case in 10 years in our hospital), we decided to present a case report of a man aged 68 with an incidentally detected skin lesion located on the scalp. Little was known about the patient as he had not been under medical supervision for over 10 years and therefore had no medical history. We only knew that he was a heavy smoker. The hairdresser found a small lump on the scalp during a haircut. Upon physical examination, a 10-mm, well-delineated, painless nodule in the hairy scalp was identified. Surgical excision of the tumour was performed under local anaesthesia, with a margin of healthy skin.
Results
This review analysis showed that genitourinary malignancies metastasised to the skin as listed in Table 1. Analysis of recent reports, based on case report analyses from Science Direct and Web of Science (2000–2019) showed that 63% of the cases involved patients with a secondary cutaneous metastasis who underwent a surgical treatment of kidney tumour in the past. As many as 37% of patients were diagnosed with skin metastases as a primary manifestation of cancer (Table 2). Based on the analysis of up-to-date case reports, CM of RCC are most often found on the face (43% of patients) followed by the scalp (30% of patients) and the neck (7% of patients). Table 3 shows the metastatic sites of the skin.
Table 1
Localization of new cutaneous lesions in groups of patients with primary genitourinary malignancy
| Primary cancer | Percent of skin metastases (2014) ref [25] | Percent of skin metastases (2004) ref [27] | Percent of skin metastases (1993) ref [29] | Common presenting location |
|---|---|---|---|---|
| Kidney | 2.7 | 3.4 | 1.4 | Head-neck |
| Bladder | 0.2 | 0.84 | 1.7 | Abdomen |
| Prostate | 0.5 | 0.36 | 0 | Abdomen |
| Testes | 0.5 | 0.4 | 0 | Head-neck, chest |
Table 2
List of RCC case reports with skin metastasis published between 2000 and 2019 (based on analyses from Science Direct and Web of Science)
| Author | Reference | Age of diagnosis | Gender | Skin metastases as the first presentation of RCC | Mets after kidney surgery for RCC | Time period between primary kidney tumour diagnosis and skin metastases |
|---|---|---|---|---|---|---|
| Bjurlin et al. | [28] | 40 | M | Yes | ||
| Gonzales et al. | [29] | 77 | M | Yes | ||
| Soares et al. | [30] | 70 | M | Yes | 5 months | |
| Cabrera-Beyrouti et al. | [31] | 87 | M | Yes | ||
| Bhatia et al. | [32] | 63 | M | Yes | ||
| Navarrete-Gutiérrez et al. | [33] | 51 | M | Yes | ||
| Nakano et al. | [34] | 72 | M | Yes | 19 years | |
| Boaz et al. | [35] | 38 | M | Yes | 1 year | |
| Jatti et al. | [36] | 60 | M | Yes | 5 months | |
| Riter et al. | [37] | 53 | M | Yes | No information | |
| Porter et al. | [38] | 36 | M | Yes | ||
| Lim et al. | [39] | 86 | M | Yes | 4 years | |
| Fernandez-Rueda et al. | [40] | 80 | M | Yes | 2 years | |
| Pritchyk et al. | [41] | 70 | M | Yes | 5 years | |
| Rajasekharan et al. | [42] | 55 | M | Yes | ||
| Tadashi et al. | [43] | 84 | M | Yes | 9 years | |
| Arrabal-Polo et al. | [44] | 73 | M | Yes | 10 years | |
| Ferhatoglu et al. | [45] | 40 | F | Yes | 14 months | |
| Kandemir et al. | [46] | 53 | M | Yes | 3 years | |
| Cui et al. | [47] | 86 | M | Yes | ||
| Mirza et al. | [48] | 41 | M | Yes | ||
| Errami et al. | [49] | 64 | M | Yes | 3 years | |
| Singh et al. | [50] | 51 | M | Yes | 11 years | |
| Abbasi et al. | [51] | 42 | M | Yes | 1 month | |
| Chauhan et al. | [52] | 57 | M | Yes | ||
| Opper et al. | [53] | 63 | M | Yes | No information | |
| Pan et al. | [54] | 63 | M | Yes | ||
| García Torrelles et al. | [55] | 60 | M | Yes | 5 years | |
| Soda et al. | [56] | 78 | M | Yes | ||
| Snow et al. | [57] | 69 | F | Yes | 6 years | |
| Kotak et al. | [58] | 64 | M | Yes | 6 months |
Table 3
Metastatic sites of the skin in RCC patients based on up-to-date case reports published between 2000 and 2019
| Location | Number | % | References | |
|---|---|---|---|---|
| Face | Lips | [26, 34, 37, 45, 56] | ||
| Eyelids | [27, 29] | |||
| Cheeks | 16 | 43 | [14, 28, 32, 37] | |
| Nose | [30, 40] | |||
| Chin | [33, 36, 38] | |||
| Scalp | 11 | 30 | [14, 35, 38, 43, 46, 47, 49, 52, 53, 54, 55] | |
| Neck | 7 | 19 | [30, 37, 41, 42, 44, 50, 51] | |
| Extremities | 3 | 8 | [13, 35, 50] | |
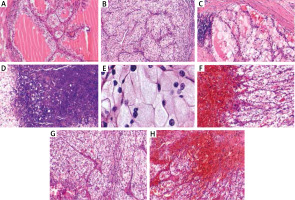
In the histopathological analysis the lesion was described as a clear cell carcinoma (Figure 1) and determined to be a renal cancer metastasis. The tumour was excised with a minimum 2 mm surgical margin. A positive result for CD10 was noted in immunohistochemical staining. The patient underwent computed tomography (CT) of the abdominal cavity and chest. It showed the primary source of metastasis to the skin, and in the superior pole of the left kidney a partially exophytic, solid mass measuring 60 × 65 mm and lacking lymphadenopathy in the retroperitoneal space. A left-side nephrectomy was performed and clear RCC was confirmed via histopathological analysis (Figure 1). The image demonstrates the optically clear tumour cells with uniform small nuclei without nucleolar ranged in an alveolar pattern (Figures 1 B, C). Slightly irregular nuclear contours; nucleoli visible (Figure 1 E). Nuclei are uniform and small with inconspicuous nucleoli at this power (Figure 1 A) Necrotic changes are visible (Figure 1 D).
Figure 1
The micrograph of a clear cell renal cell carcinoma haematoxylin and eosin (H&E) staining obtained from the described patient. Skin metastasis of renal cell carcinoma H&E sections (F–H). Scale bar: A–D and F–H 200 μm; E – 50 μm. The samples were analysed morphologically and photographed under an Olympus BX43 light microscope equipped with an Olympus SC50 digital camera
According to the Memorial Sloan-Kettering Cancer Centre (MSKCC) Risk Group, this case was described as the intermediate risk group. A clinical and radiological follow up at 30 months did not show tumour recurrence.
Discussion
RCC has been classified as the most common kidney tumour and its morbidity has increased from 1.5% to 5.9% annually [8]. Cutaneous metastases are seen in approximately 2.8–6.8% of RCC cases. According to the statistics, skin metastasis had been observed before a primary tumour in 16–21% of cases. However, the prevalence has been reported as high as 26% [6, 9]. It is said that cutaneous metastases might be the first signs of clinically silent visceral cancer [10]. Analysis of recent reports shows that the mean period of time between a nephrectomy and diagnosed cutaneous metastasis was 4.5 years, with the longest period reported to be 19 years [9]. These reports also revealed that 94% of cases were male. In the present case, RCC was incidentally found before the primary tumour was detected in the kidney during a visit to a hairdressing salon.
Paolino et al. described the results of 118 reports for 123 cases with alopecia neoplastic from visceral tumours. It was observed that the most common place of the primary tumour was the gastrointestinal tract, followed by breast, kidney, lung, thyroid, uterus, central nervous system, and liver [11]. The authors indicated that in the patients with localized scarring alopecia one always ought to remember about metastatic skin disease [11].
The most frequent site for skin metastasis of RCC has not been found yet. Dorairajan et al. indicated that in 50% of RCC cases, the cutaneous metastasis was diagnosed on the scalp [12]. In turn, observations made by Koga et al. suggested that 40% of skin metastases cases occurred on the trunk, and only 25% on the scalp [13]. Additionally, up to 75% of patients had secondary metastases in at least one other site, most commonly in visceral organs, such as the lungs and liver [14]. Distant organ metastases from the primary focus most often occur through lymphangiogenesis. The second way to form distant metastases is the vascular route associated with the increased process of angiogenesis, which is observed in neoplasms [15].
In our case, the metastatic skin lesion was about 10 mm, with red-purple colour, and a nodular type. Skin metastasis of RCC may be confused with haemangioma, pyogenic granuloma, Kaposi’s sarcoma, infected skin cysts, or skin lymphoma, and for that reason, we should consider those lesions in the differential diagnosis [16, 17].
The large acinar structures formed from clear RCC have layers of clear cells with central glassy eosinophilic secretions (Figures 1 A–E). Immunohistochemically, 60% of RCC tumour cell skin lesions express vimentin, epithelial membrane antigen (EMA), carcinoembryonic antigen (CEA), CD10, RCC-Ma, and keratins [3]. Their development became possible thanks to the development of methods and techniques of molecular biology. Currently, there are databases available containing sets of genes and their coding proteins characteristic for a given tumour [18].
The prognosis of the patients with RCC metastases is unfavourable. In general, the five-year survival rate for the patients with RCC solitary metastases is 10–13% [19]. The average life expectancy usually is less than 6 to 12 months from diagnosis. It should be noted that 5-year survival increases by approximately 30-45% in patients with RCC and metastases who underwent metastasectomy [20].
The treatment recommendation in skin metastases of RCC is surgery (nephrectomy and metastasectomy). Surgery in advanced patients may be completed by tyrosine-kinase inhibitor therapy. It has also been suggested that radiotherapy with adjuvant chemotherapy could have good results. Gay et al. noticed that complete remission was observed in a patient with a solitary skin metastasis after both sorafenib therapy and radiotherapy [21]. Lyon et al. showed that in patients with a complete metastasectomy, in comparison to patients without, 2-year CSS was significantly greater, what was related with reduced probability of death due to RCC (p < 0.001) [20]. Ouzaid et al. observed lower mortality in patients who underwent metastasectomy compared to those who did not [22, 23]. In turn, Tosco et al. suggested that the Leuven-Udine classification ought to be used in analysis of the results of surgically treated patients [24]. Summarizing, cutaneous metastases of RCC are indicative of a poor prognosis, but disease-free follow up is possible after early diagnosis and the appropriate surgical excision. Based on the analysis of up-to-date case reports, cutaneous metastases of RCC are most often found on the face. Relying on systematic reviews, nephrectomy and complete metastasectomy are viable therapeutic options, resulting in significantly improved CSS and reduced likelihood of death from RCC. Furthermore, the presented case highlights the importance of oncological diagnostics such as multiple detailed physical examinations as they are critical to positive patient outcomes to supplement the continuous development of new imaging techniques and laboratory diagnostics.








