Introduction
Psoriasis is a chronic, inflammatory, and debilitating condition which may affect skin and joints [1, 2]. The disease occurs in between 0.51% and 11.43% of population, depending on the region of the world [3]. Treatment with biologics was a breakthrough that allowed doctors to control the disease effectively, giving majority of the patients better quality of life [4].
Biological medications act on selected elements of immune response and are registered for moderate to severe forms of psoriasis and psoriatic arthritis [2]. The clinical experience to date confirms that, in addition to its efficacy and rapid response, biological therapy is safe and well tolerated [1, 2]. Currently, guidelines to match the best type of biologic to the individual psoriatic patient’s need are being developed [1, 5].
Many medical conditions display seasonality in terms of presenting with a first episode or exacerbation of an existing medical problem [6–11]. Clinical observations pointed to the same pattern in psoriasis with deterioration or first episodes starting mainly in cold months [12–14]. This pattern was not however observed by all authors, citing multifactorial nature of the disease [15, 16]. Recently, a review on seasonal variation of psoriasis in Northern and Central Europe revealed that 50% of patients showed no difference in the severity of their skin lesions, while 30% of them reported improvement in summer months and 20% in winter season [17].
Our clinical observations suggested a different course of clinical response in psoriatic patients depending on starting time of treatment (winter and summer) and the type of biologics used.
Aim
Therefore, the aim of this preliminary, observational study was to test the hypothesis of better outcome of the treatment with biological medications in patients with psoriasis starting therapy in the warm period of the year comparing to the cold period and to identify whether the difference, if existed, depended on the type of biologics used.
Material and methods
Anonymized data of 62 adult patients with moderate to severe plaque-type psoriasis, treated with biological medications at two dermatology departments were retrospectively examined. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the Medical University of Lodz (protocol code RNN/179/20/KE, 14 July 2020).
The patients were treated with adalimumab, infliximab, ustekinumab, secukinumab, and ixekizumab (31, 14, 7, 4, and 6 patients, respectively). Adalimumab and infliximab are inhibitors of tumor necrosis factor-α (TNF-α) while ustekinumab, secukinumab and ixekizumab are inhibitors of other proinflammatory cytokines (IL-k, k = (12/23 and 17)). These two groups of biologics are hereinafter referred to as TNF-α and IL-k, respectively. To compare differences of the patients’ response to the treatment according to the therapy starting point, two groups of patients were chosen: those beginning the treatment in the cold period of the year (November–March) (23 patients, 7 TNF-α and 16 IL-k) and in the warm period (May–September) (34 patients, 12 TNF-α and 22 IL-k). The number of recruited patients was not large enough to make a month-by-month data split. Further in the text, the former group will be called the “winter” group and the latter the “summer” group.
The psoriatic severity and quality of life scores, Psoriasis Area and Severity Index (PASI), Body Surface Area (BSA) and Dermatology Life Quality Index (DLQI), were recorded at the beginning, after 1, 4, and 7 months of the therapy. Outcomes of psoriasis treatment in terms of changes of PASI, BSA and DLQI after each time point of therapy were analysed for the whole winter and summer groups and separately for TNF-α and IL-k groups. In addition, PASI75 and PASI90 scores were also analysed, which indicate the proportion of patients achieving at least 75% and 90% reduction in PASI score from baseline, respectively.
Statistical analysis
The Wilcoxon rank-sum test was chosen for testing significance of the differences between various subgroups of the patients, i.e., “winter” and “summer” patients, those using TNF-α and IL-k. In addition, Fisher’s exact test was used when analysing the 2x2 contingency tables. The analytic software package MATLAB 2018a (MathWorks, Inc.) was used for these statistical tests.
Results
Table 1 provides characteristics of the winter and summer groups of patients at the beginning of the therapy. There were no statistically significant differences between the groups. Similarity between the winter and summer groups before starting biologics can also be seen when comparing the first pair of boxes in each part of Figure 1. It is worth mentioning that the lowest score was slightly above 10 for PASI and BSA, but the lowest score for DLQI was 7. Figure 1 C shows that this value could be classified as the outlier and the smallest reliable DLQI value should be also above 10.
Table 1
Statistical characteristics (median ± standard deviation of median) of the winter and summer groups at the beginning of the treatment and p-values for the hypothesis of no difference between variables in winter and summer groups
Figure 1
Box plots for psoriasis severity characteristics in the winter and summer groups: PASI (A), BSA (B), DLQI (C) for the winter (white boxes) and summer (grey boxes) groups at the start of the treatment, after 1, 4 and 7 months. Edges of boxes are 25th and 75th percentile, the horizontal line in the middle is a median, while whiskers are minimum and maximum. Crosses are outliers
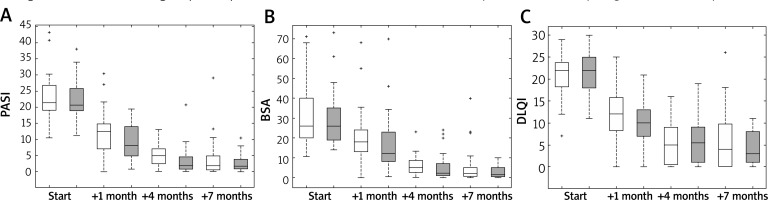
Table 1 shows also statistical characteristics of the winter and summer groups after 7 months of therapy. P-values suggest that there were insignificant differences between outcomes for these groups at the end of therapy. Psoriasis improvement was obvious by comparing the starting and final outcomes. This could be also inferred from Figure 1 (see the last pair of the boxes located well below the first pair). The significant improvement of psoriasis activity is also seen in Figure 1 after 1 month and 4 months but there is only a minor improvement of psoriasis scores between 4 and 7 months since the start.
Figure 2 presents changes in psoriatic scores after each time point of the therapy for the winter and summer patients, further divided into two categories of biologics used (TNF-α and IL-k). For all psoriasis severity scores, the rate of improvement seemed to be the greatest after the first month of therapy for the summer group treated with IL-k biologics, decreased at subsequent two time points and was the lowest at the end of therapy.
Figure 2
The same as Figure 1 but box plots are for the winter and summer patients treated with biologics: IL-k inhibitors and TNF-α inhibitors
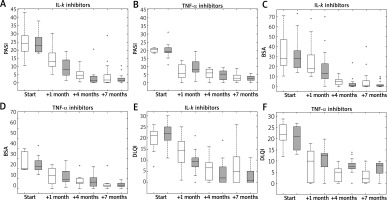
Table 2 provides p-values for the outcomes of treatment depending on the type of biologics used (IL-k versus TNF-α) and the starting point of the therapy (winter versus summer). It is worth noting that there is a correlation between the results presented in Table 2 for the column “no division” and the differences between the position of box plots of different colours (white/grey) in Figure 1 at each stage of therapy. Moreover, there is a correlation between results in columns “IL-k” and “TNF-α” and box plots on the left- and right-hand side of Figure 2, respectively. For example, regarding the DLQI score after 7 months of TNF-α inhibitor therapy, the grey (summer) box plot in Figure 2 F is above the white (winter) box plot at the end of therapy, suggesting better performance of the winter group. This difference in position of the box plots is statistically significant as given by p-value of 0.03 in Table 2, i.e., the last value in the column “TNF-α”. However, this improvement was not confirmed by the PASI and BSA results.
Table 2
P-values by Wilcoxon rank-sum test for differences in the therapy between the seasonal groups. Results are also for the groups without (column “no division”) and with division into two categories of biologics used (column “IL-k” and “TNF-α”)
For the summer group comprising all patients starting in summer (“no division“ group), better outcome of the therapy was revealed for BSA after 1 and 4 months. This was also found for PASI, but only after 4 months. Results for PASI and BSA in seasonal groups with division into the type of biologics used showed that a better outcome of the overall summer group was due to IL-k biologics. For the patients treated with IL-k inhibitors, a better outcome of the summer group was also found in PASI and BSA (after 1 and 4 months), and in DLQI (after 1 month). A better outcome for the winter group was revealed in DLQI values for patients using TNF-α inhibitors but this appeared after 4- and 7-month therapy.
Table 3 shows that PASI75 and PASI90 were usually higher in the summer group except scores after 1 month therapy with TNF-α inhibitors. The largest difference between the seasonal groups were after 4-month therapy with IL-k. But after 7 months, the differences between the groups decreased significantly. The significance of differences between summer and winter groups were assessed using Fisher’s exact test with 2x2 contingency table (Table 4). After 4 months, better outcomes (with p-value ≤ 0.05) in the summer group were identified based on PASI75 and PASI90 scores in patients treated with IL-k. This was also found for PASI90 score for the “no division” group.
Table 3
PASI75 and PASI90 calculated after 1-, 4- and 7-month therapy for the winter and summer groups without and with division into two different categories of biologics used by the patients
Table 4
P-values by the Fisher exact test for no difference in PASI75 (and PASI90) scores between summer and winter groups. The results are calculated for each step of therapy without (column “No division”) and with division into biologics used (columns “IL-k” and “TNF-α”). Statistically significant values are in bold
Discussion
Clinically observed seasonality of medical conditions is a well-known phenomenon [18]. There is a common belief that in majority of patients with psoriasis, skin lesions improve in warmer months of the year [18–20]. The study by Ferguson et al. [19] revealed that 77% of patients with psoriasis reported seasonal changes in the disease activity with exacerbations in winter (67%) and summer months (24%). Internet searches connected with psoriasis and its treatment also showed statistically significant seasonality peaks in late winter and lowering in summer. This could theoretically on its own explain the results in our study. However, not all literature data support this statement. Brito et al. [7] examined seasonality of the hospitalizations at a dermatological ward with no differences observed regarding patients with psoriasis and Kubota et al. [21] did not observe any difference in seasonal frequency in the number of patients with psoriasis using health service in Japan. A systematic review on psoriatic patients in Northern and Central Europe showed that 30% of patients were better in summer months and 20% in winter [17]. Therefore, the differences observed in our cohort cannot be in our opinion entirely explained by seasonality of psoriasis course.
Outdoor conditions for the mid-autumn/winter and mid-spring/summer seasons are very different in Poland with higher temperature and stronger solar radiation in the latter period. In mid-spring/summer the patients have a chance to synthesize a large amount of vitamin D during outdoor activities due to UV-B (290–315 nm) exposure. They also receive more solar radiation in UV-A (315–400 nm) and visible range (400–700 nm), and prolonged outdoor exposures until late in the evening could interfere with melatonin production. Moreover, it cannot be excluded that other, yet unknown biological and environmental factors affect medical treatment [22].
The follow-up period in our group of patients was 7 months. This means that the “winter” patients also met the summer season, and vice versa, “the summer” patients met the mid-autumn/winter season. Statistical tests showed a better outcome (for all psoriatic metrics) for the “summer” group after the first month of the therapy with IL-12/23 and IL-17 inhibitors. Such improvement was also present in PASI and BSA scores after 4 months of the therapy. At the end of treatment, outcomes were similar in all groups. However, the TNF-α inhibitor group showed a gradual improvement in the psoriasis outcomes without a sudden increase in efficacy in the first months of therapy, as observed in the summer group treated with IL-k inhibitors.
There were no vitamin D measurements obtained in this group of patients as it is not routinely tested in patients on biologics, however a large population-based study did not reveal any correlation between psoriasis, active psoriasis and vitamin D levels [23]. Meta-analyses did not support a positive effect of supplementing vitamin D in psoriatic patients [24, 25]. The Medical Board of the National Psoriasis Foundation in the USA does not recommend oral vitamin D in psoriatic patients with a normal vitamin D level [26]. The improved healing of psoriasis observed only in the first months of therapy in the summer group treated with IL-k inhibitors does not support the possible influence of the patients’ vitamin D status on the outcome of biologic therapy.
Considering other environmental factors, it has been reported that changes in humidity are significantly influencing the structure of epidermis and can contribute to seasonal deteriorations and improvements of inflammatory dermatoses such as atopic dermatitis and psoriasis [27]. Liang et al. [28] in their retrospective study found a correlation between the initiation of systemic therapy and humidity of the geographical area the patient lived in. Another possible explanation to the phenomenon is seasonally fluctuating expression of genes. Dopico et al. [6] examined over 22,000 protein-coding mRNAs and demonstrated that ~23% of them showed differences in expression depending on the time of year. Moreover, they observed an inverted pattern of those expressions when comparing Europeans to Oceania people. Among others, during winter months in Europe, a proinflammatory profile with high levels of soluble IL-6 receptor and C-reactive protein was observed. However, our study showed that the difference in psoriasis clearance between the summer and winter groups, which was identified at the initial stages of the treatment, depends on the biologic type used in the therapy.
The available literature data showed that the initiation of systemic medications, including biologics, was the highest in spring and switching between the medications was higher in spring and summer [28]. Ruano et al. [29] analysed a group of patients with moderate to severe plaque psoriasis treated with TNF-α inhibitors who after achieving a significant clinical response had a temporary suspension of the treatment. The authors found out that the risk of relapse and duration of the remission were related to the time of the year the treatment was stopped.
The weaknesses of our study include relatively small numbers of the patients. We are in the process of gathering more data to further investigate this issue. The information on the natural course of the disease before the start of the biologic therapy in relation to the time of the year was not available. That data would be difficult to obtain anyway as all patients with moderate to severe psoriasis were on systemic treatment for long periods of time before being able to qualify for the treatment with biologics due to national guidelines. Considering the findings of Jensen et al. [17] that only 30% of psoriatic patients in Northern and Central Europe, a geographically similar area to that of our patients, reported improvement in their disease activity in summer months, it seems that some other factors could be responsible for results obtained in the study. More data are needed whether in our patients the differences in efficacy of the biologic therapy were connected to the seasonality of the disease itself or to the treatment received. The good news for the patients is that over time the differences in efficacy levelled up.
Conclusions
A clear sign of seasonality appeared in the effectiveness of IL12/23 and IL17 inhibitors therapy in moderate to severe psoriasis with better results obtained within first months of treatment in patients starting therapy in the warm period of the year (May-September). The course of psoriasis improvement was different in patients treated with TNF-α inhibitors, where a steady improvement was observed, regardless of environmental factors. The outcome after 7 months of treatment did not show significant differences in effectiveness of the treatment between the two groups of medications. This is potentially very important information for the patients who expect quick improvement on biologics. Informing them that it may take longer to achieve significant improvement when starting treatment in winter months, but the overall effect will be the same, may therefore improve their compliance. Our understanding of not only the chronobiology of psoriasis as such, but also of the effectiveness of the treatments depending on the time of the year combined with the type of biologics used, may further improve the results of the therapy.








