Introduction
Thymoma, originating from thymic epithelial cells, is the most common anterior mediastinal tumor, with an annual incidence of 2.5 per 100,000 people [1]. Thymoma is closely related to autoimmune diseases, the most common of which is myasthenia gravis (MG). Thymectomy is an important method for the treatment of MG in clinical practice. A randomized controlled study published in “The New England Journal of Medicine” in 2016 provided a strong basis for thymectomy in the treatment of MG [2], but the molecular mechanisms of thymoma leading to MG still need to be further explored.
Current studies suggest that Th17 cells play a role in the pathogenesis of autoimmune diseases and mediating chronic inflammation, while Treg cells maintain immune tolerance by inhibiting the activation and proliferation of CD4+ T cells. Th17 cells and Treg cells jointly maintain the relative stability of the immune function through their mutual antagonism in differentiation and function, and the imbalance of them may be a key factor in the pathogenesis and development of a variety of autoimmune diseases and inflammatory diseases [3-5]. Our previous studies showed that the expression of Th17 cells and related cyto- kines (IL-6) increased and the expression of Treg and Foxp3 decreased in MG-associated thymoma tissues and peripheral blood [6, 7], but the mechanism of Th17 and Treg expression differences in thymoma was not elucidated.
High mobility group protein 1 (HMGB1) is a non-histone protein mainly located in the nucleus and promotes the nuclear transcription process by interacting with DNA. When the body has tumors or immune abnormalities, cells will secrete HMGB1 as a “danger signal”, and the secreted HMGB1 may also trigger tumor progression. HMGB1 is an evolutionarily conserved nuclear protein that binds to DNA to maintain chromatin structure, participates in DNA repair, and indirectly regulates the activities of various transcription factors such as NF-κB and the glucocorticoid receptor [8, 9]. HMGB1 also participates in various extracellular activities as an endogenous danger signal or damage associated molecular pattern (DAMP). It is involved in cell-cell interaction, including production of proinflammatory cytokines, cell proliferation, differentiation, invasion, and autophagy [10]. Studies have confirmed that HMGB1 is associated with the development of a variety of autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis and immune vasculitis. Moreover, HMGB1 has a critical effect on the differentiation and maintenance of Treg and Th17 cells.
The discovery of the proinflammatory properties of HMGB1 and the role of extracellular HMGB1 in autoimmune diseases quickly highlighted it as a potential therapeutic target for inflammatory and autoimmune diseases. HMGB1 is involved in the pathogenesis of the passive transfer MG model, suggesting that HMGB1 plays a role in the exacerbation of inflammation at the MG neuromuscular junction [11]. However, as the most common central immune organ in MG, whether thymus is involved in the occurrence and development of MG is still lack of corresponding research. Therefore, the present study aimed to investigate the mechanism.
Material and methods
Patients and samples
A total of 50 patients with thymoma were included in this study. All patients were pathologically diagnosed with thymoma, including 20 cases of thymoma and 30 cases of thymoma with myasthenia gravis. Thymoma tissue samples were collected during surgery, and a portion of the tissue was fixed in 10% formalin, followed by paraffin embedding. The study was approved by the Ethics Committee of Tianjin Medical University General Hospital.
Peripheral blood lymphocyte subsets were detected by flow cytometry
Flow cytometry results of clinical laboratory data were counted, including CD20, CD19, CD3, CD8, Th17 (CD4+IL-17+), Treg (CD4+CD25+Foxp3+), Tfh (CD4+ CXCR5+IL21+), Tfr (CD4+CXCR5+Foxp3+). Immune indicators include immunoglobulin (Ig)G, IgA, IgM, IgE, C3, C4, C-reactive protein (CRP), circulating immune complex and antinuclear antibody.
Immunohistochemical staining of HMGB1 in thymoma
Immunohistochemical (IHC) staining was performed on paraffin-embedded thymoma tissue sections (4 µm) with anti-HMGB1 antibody (ab79823, dilution 1 : 400, Abcam). IHC staining was performed according to the manufacturer’s instructions of the IHC staining kit (PV-6000, Beijing Zhongshan Goldenbridge Biotechnology).
Cell culture
The thymoma cell line Thy0517 was stored at the cardiothoracic surgery department of Tianjin Medical University General Hospital. Thy0517 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). Peripheral blood mononuclear cells (PBMCs) were isolated from healthy volunteers by gradient centrifugation with Lymphocyte Separation Medium (Solarbio, China). PBMCs were cultured in RPMI 1640 supplemented with 10% FBS and 25 µl Human CD3/CD28 T Cell Activator (STEMCELL, 10971, USA). All cells were incubated in a 5% CO2 atmosphere at 37oC.
RNA interference
HMGB1 silencing was performed by infecting Thy0517 cells with siRNA (GenePharma, China). The sequence of si-HMGB1 is 5´-CUGUCCUUGAAGGGACUAAUTTAUUAGUCCUUCAAGGACAGTT-3´. The si-HMGB1 and negative sequence were transfected into Thy0517 cells using Lipofectamine 2000 Transfection Reagent (Invitrogen, USA) according to the manufacturer’s protocol. After 48 h, cells and cell supernatants were harvested for subsequent experiments.
Serum IL-6 ELISA assay
Cell supernatants were centrifuged at 1500 rpm for 5 minutes. The concentrations of IL-6 in the cell supernatants were determined with the Human IL-6 Precoated ELISA kit (DAKEWE, 1110602, China) according to the manufacturer’s instructions.
Co-culture of RNA interfered Thy0517 and PBMCs
Co-culture of RNA interfered Thy0517 and PBMCs was performed using the Transwell system (0.4 µm pore size, Corning, USA). PBMCs were added to the top portion, while an equal volume of RNA interfered Thy0517 was added on the bottom. PBMCs were harvested after three days for flow cytometry. This experiment contains two groups: si-HMGB1 and control.
Flow cytometry
To detect Th17 cells, co-cultured PBMCs were supplemented with 2 µl/ml cell stimulation cocktail (eBioscience, USA) and stimulated in a 5% CO2 incubator at 37°C for 4.5 h. PBMCs were labeled with CD4 antibodies for 20 min at room temperature. Following fixation and permeabilization, FOXP3 and IL-17A intracellular staining was performed. Treg analysis was performed with FITC-labeled anti-human CD4, APC-labeled anti-human CD25 and PE-labeled anti- human FOXP3 antibodies (Miltenyi Biotec). The stained cells were analyzed by flow cytometry (FACSCanto II; BD Biosciences, San Jose, CA, USA). Data analysis was performed using Cell Quest software (BD Biosciences).
Statistics
Statistical analysis was performed using GraphPad Prism version 8.0.2. The data are shown as mean ±standard deviation. The normal distribution test was conducted before the T-test of the data, specifically through the GraphPad Prism software. Comparisons were made using Student’s t-test. P < 0.05 was considered as statistically significant.
Results
Patients’ characteristics
A total of 50 patients with thymoma were included in this study, including 20 patients with thymoma (T group) and 30 patients with thymoma with MG (T + MG group). There were 7 males (35.0%) and 13 females (65.0%) in the T group, aged 30-74 years. In the T + MG group, there were 12 (40.0%) males and 18 (60.0%) females, aged 21-76 years. There were no significant differences between the two groups in terms of gender and mean age. There was a difference in World Health Organization (WHO) pathological staging between the two groups of thymoma patients (p < 0.05) (Table 1).
Peripheral blood lymphocyte subsets and immune protein analyses in patients with thymoma
The percentage of Th17 cells in the T group was 1.589 ±0.5798%, which was lower than that in the T + MG group (2.485 ±1.536%), and the difference was statistically significant (p < 0.05). The percentage of Treg cells in the T group was 2.842 ±1.713%, which was higher than that in the T + MG group (1.812 ±0.9560%), and the difference was statistically significant (p < 0.05). The IgG level in the T group was lower than that in the T + MG group, and the difference was statistically significant (p = 0.0005) (Table 2). Complement C3, C4 and CRP levels were higher in the T group than in the T + MG group, and the difference was statistically significant (p < 0.05). The results of serum protein examination showed that α1 globulin and γ globulin were higher and α2 globulin was lower in the T + MG group compared with the T group, and the difference was statistically significant (Table 3).
Table 2
Peripheral blood lymphocyte subset analysis of thymoma patients
Table 3
Peripheral blood immune protein analysis of thymoma patients
Expression of HMGB1 in thymoma of T and T + MG group
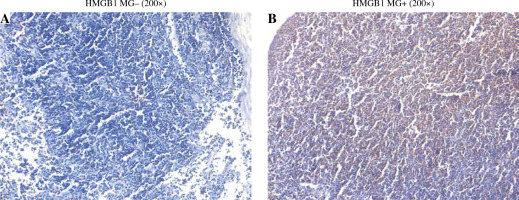
IHC staining showed that HMGB1 was expressed in both the T group and the T + MG group. The expression was lower in the T group and was mainly nuclear; the expression was higher in the T + MG group and was mainly secretory (Fig. 1).
Interleukin 6 secretion was decreased in Thy0517 after transfection with si-HMGB1
Compared with the control group, the IL-6 content in the supernatant of Thy0517 cells after transfection with si-HMGB1 was 107.4 ±0.7099 pg/ml, which was significantly lower than that of the control group (131.6 ±0.3620 pg/ml). The difference was statistically significant.
Effect of Thy0517 transfected with si-HMGB1 on differentiation of T cells
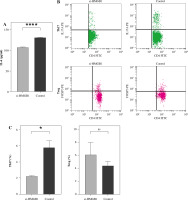
Peripheral blood mononuclear cells were co-cultured with Thy0517 transfected with si-HMGB1, and were collected after 3 days for flow cytometric detection of Th17 cells and Treg cells, and the results are shown in Figure 2B-C. The percentage of Th17 cells in the si-HMGB1 group was 2.230 ±0.07937%, which was lower than that in the control group (5.780 ±0.8106%) (p < 0.05). The percentage of Treg cells in the si-HMGB1 group was 6.133 ±1.864%, which was higher than that in the control group (4.480 ±0.6300%), but the difference was not statistically significant.
Discussion
Thymoma is the most common anterior mediastinal tumor, derived from thymic epithelial cells, with an annual incidence of 2.5 per 100,000 people [1], and the incidence is increasing. Thymic epithelial cells are critical for establishing immune tolerance to T cells. Current theories suggest that thymoma lacking corresponding structure of medulla, thymic epithelial cells and lymphatic tumor cells has a function similar to that of the normal thymus tissue, so the thymic tumor cells enter the circulation directly and cannot mature by selecting the medulla, and thymic lymphocytes lack the ability to induce immune tolerance. As a result, immature thymic lymphocytes in the thymoma mistake their own antigens for heterologous antigens and fight against them, producing corresponding autoantibodies and leading to autoimmune damage or disease [12]. The most common of these diseases is MG, in which thymic enlargement or tumors are found in 60% of patients, and thymoma is present in 15% of patients.
The results of this study show that thymoma with non-MG autoimmune diseases are more likely to occur in female patients, and the positive rate of antinuclear antibody is higher in female thymoma patients, which is consistent with the higher incidence of multiple autoimmune diseases in women. Some patients have no clinical manifestations of autoimmune diseases, but have positive expression of antinuclear antibodies. These patients may avoid the occurrence of autoimmune diseases after thymoma resection. From the perspective of the WHO pathological classification of thymoma, type B thymoma is more likely to be complicated with MG. The positive rate of antinuclear antibody was not correlated with the WHO pathological classification of thymoma. This result is consistent with many reports [13, 14], because the thymic epithelial cells expressing HLAII are less numerous or absent in type B thymic tumors, and AIRE and/or myoid cells are largely absent, and their striated muscle autoantigens are not tolerated by DC cell-mediated cross-tolerance [15].
The results of this group of immune indicators showed that the meaningful indicators included IgG, C3, CRP and circulating immune complexes. IgG is the major antibody component of serum and is elevated in a variety of autoimmune diseases [16]. C3 is the most abundant complement component in serum. In autoimmune diseases such as systemic lupus erythematosus, excessive complement depletion or loss can lead to the reduction of C3 [17]. The autoantibodies of acetylcholine receptor (AChR) are polyclonal IgGs (mainly IgG1 and IgG3) that can react highly specifically with various AChR epitopes. According to Paz and Barrantes, some autoantibodies induce postsynaptic membrane cleavage through complement activation, and they also promote the degradation and internalization of AChRs [18]. The decrease of serum C3 in MG patients in this study indicates that C3 plays an important role in the pathogenesis of MG and can be used as an important detection index in the process of disease diagnosis and treatment. CRP can reflect the activity of immune diseases. The CRP content of MG patients in this group was decreased, which may be because some MG cases were ocular type, with a mild autoimmune reaction and no significant increase in CRP, or because many patients were already receiving immunotherapy at the time of the test, leading to a drop in CRP.
The abnormal structure and function of the thymus caused by thymoma may lead to T cell receptor gene rearrangement and affect T cell differentiation. Theoretically, some changes of T lymphocyte subsets should be detected in patients with thymoma, but the results of this study showed that the expression level of Thl7 cells in peripheral blood of MG patients was increased, the expression level of Treg was decreased, and the Thl7/Treg value was significantly increased, which was not the case in patients with thymoma alone. This indicates that the balance between Thl7 and Treg cells is more important, and the imbalance between them may be an important factor in the pathogenesis and development of a variety of autoimmune diseases and inflammatory diseases, such as autoimmune diseases arthritis, sarcoidosis, psoriasis, systemic lupus erythematosus, inflammatory bowel disease, etc. [19]. Similar results have been observed in animal studies. In experimental autoimmune myasthenia gravis mice, the balance between Th1, Th2, Th17 and Treg was disrupted, with increased expression of Th1 and Th17 cells and decreased expression of Th2 and Treg cells. With the progression of the disease, the proportion of Th17 cells changed most significantly, and the expression of IL-17 was up-regulated. In response to IL-17 stimulation, the proliferation of T-cell-specific AChR peptides was enhanced, and the number of cells secreting anti-AChR antibodies was increased [20].
Thymectomy is an important method for the treatment of MG. A randomized controlled study published in “The New England Journal of Medicine” in 2016 provided a strong basis for the treatment of MG by thymectomy [2]. However, the basic research on thymoma is relatively sparse, and the molecular mechanism of myasthenia gravis caused by thymoma is not completely clear. HMGB1 is expressed in immune cells and a variety of cells, and can affect Th cell differentiation by activating DC cells or directly acting on CD4+ T effector cells. Therefore, we hypothesized that activation of HMGB1 signaling in thymoma may cause imbalance of Th17/Treg expression and induce MG.
Chromatin binding non-histone HMGB1 is released from the nucleus into the extracellular environment in specialized environments such as autoimmunity, sepsis, and hypoxia. Extracellular HMGB1 is involved in pattern recognition receptors, including Toll-like receptors (TLRs) and receptors for advanced glycosylation end products (RAGE). Tissue staining in this study also suggested that HMGB1 was secretively expressed in a compound classical mode of action. Because HMGB1 plays an active role in inflammation and immune responses as an endogenous alarm protein, many groups have focused on its therapeutic potential by inhibiting its gene expression, cellular release, or extracellular activity as a cytokine/chemokine [21]. In many types of cancer, including non-Hodgkin’s lymphoma, melanoma, gastric cancer, cervical cancer, breast cancer, osteosarcoma, and mesothelioma, HMGB1 overexpression and/or high serum HMGB1 were found [22] and 18 studies involving 11 cancers were systematically reviewed. HMGB1 overexpression was associated with poor prognosis [23]. In addition, HMGB1 stimulated the release of inflammatory cytokines, which mediate a systemic inflammatory response and local tissue damage [24]. HMGB1 is associated with a variety of autoimmune diseases, and is positively correlated with the severity of the disease [25]. In this study, it was proved that the expression level of HMGB1 was higher in thymoma tissues complicated with MG. These results suggest that HMGB1 may play a key role in the development of the inflammatory process in MG pathogenesis. Previous studies have also suggested that myasthenia gravis occurs more in type B2 thymoma, and HMGB1 in tumors is related to the prognosis and invasiveness of tumors. Therefore, it is speculated that relatively severe thymoma leads to the occurrence of MG.
In MG patients, disruption of the neuromuscular junction by the complement system and AChR antibodies may lead to the production of HMGB1, causing severe inflammation and further disruption of the neuromuscular junction. However, HMGB1 is involved in the acquisition of passive transfer myasthenia gravis model. However, the clinical laboratory data of patients in this study suggest that there were significant differences in C3 and C4 between the simple thymoma and thymoma with MG groups, which verified this theory.
Autoreactive CD4 T helper cells (Th) are closely related to the progression of experimental autoimmune myasthenia gravis (EAMG) and MG, because they secrete cytokines and trigger the production of pathogenic autoantibodies by autoreactive B cells. Th17 cells and Treg cells jointly maintain the relative stability of the body’s immune function through their mutual antagonism in differentiation and function. The imbalance between Th17 and Treg may be a key factor in the pathogenesis and development of a variety of autoimmune diseases and inflammatory diseases [4-6].
The proinflammatory effect of Th17 and the inhibitory function of Treg cells are related to the pathogenesis of many autoimmune diseases. Recent studies have shown that the imbalance between the expression of Th17 cells and Treg cells is the common mechanism of many autoimmune diseases. The development of therapeutic drugs that regulate various molecular targets on the Th17/Treg axis and regulate the Th17/Treg balance has also become a new direction for the treatment of autoimmune diseases in recent years.
Current studies suggest that Th17 cells play a role in the pathogenesis of autoimmune diseases and mediating chronic inflammation, while Treg cells maintain immune tolerance by inhibiting the activation and proliferation of CD4+ T cells. Therefore, Th17 cells and Treg cells play an important role in the induction of central and peripheral immune tolerance. The imbalance of Th17 and Treg expression has also been one of the research hotspots in many autoimmune diseases, including MG, in recent years. However, whether thymoma can cause the imbalance of Th17/Treg expression and the mechanism of the imbalance are not very clear at present.
Interleukin 6 plays a key role in the differentiation of the two cell types. TGF-β can induce the differentiation of naive T cells into Th17 cells or Treg cells, but the differentiation of Treg cells is inhibited in the presence of IL-6, and a large number of Th17 cells are produced. Therefore, abnormal regulation or excessive production of IL-6 will lead to the occurrence of autoimmune diseases. IL-6-deficient mice showed significant resistance to EAMG development, reduced production of anti-AChR antibodies, reduced AChR-specific lymphocyte proliferative responses, and reduced expression of interferon γ (IFN-γ) and IL-10 [26].
Studies have found that HMGB1 is an upstream factor of TLR4 in a variety of diseases, which can activate the expression of TLR4, and then affect the imbalance of Th17/Treg expression, and affect the occurrence and development of diseases.
HMGB1 can cause a variety of autoimmune diseases by inducing the production of inflammatory factors or the expression changes of Th17/Treg cells. HMGB1 is highly expressed in peripheral blood of MG patients, and HMGB1 is positively correlated with the condition of MG. The expression of HMGB1 in MG patients with thymoma is higher than that in MG patients without thymoma [27].
The functional defect of Treg cells is an important mechanism leading to autoimmune MG, and the main reason is the loss of expression of Foxp3, a transcription factor that plays an important role in the function and development of Treg cells, which is related to the severity of MG [28]. Th17 cells also play an important role in the pathogenesis of MG. By producing IL-17, Th17 cells promote the increase of IgG2b expression level and the loss of B cell tolerance, which is the mechanism of AchR-specific antibody production [29]. In EAMG mice, it was found that the balance between Th1, Th2, Th17 and Treg was broken, the expression of Th1 and Th17 cells was increased, and the expression of Th2 and Treg cells was decreased. With the progression of the disease, the proportion of Th17 cells changed most significantly, and the expression of IL-17 was also up-regulated. Under the stimulation of IL-17, the proliferation ability of T cell-specific AChR peptide was enhanced, and the number of cells secreting anti-AchR antibody was increased [20].
Previous results showed that serum HMGB1 level was significantly increased in MG patients with anti-AchR antibody positive. In addition, these levels tended to correlate with the MG phenotype and declined after treatment [27].
Conclusions
Peripheral blood IgG levels were higher in patients with thymoma combined with MG than in patients with thymoma only, and C3, C4 and CRP levels were lower than in patients with thymoma only. The serum levels of α1 globulin and γ globulin were higher and α2 globulin levels were lower in patients with thymoma combined with MG compared to patients with thymoma alone. The above peripheral blood immunological and serological examinations suggest that the immune status of patients with thymoma combined with MG is different from that of patients with thymoma alone.
HMGB1 expression was higher in tumor tissues of patients with thymoma with MG than in patients with thymoma only. Downregulation of HMGB1 in thymoma cell line Thy0517 could reduce the secretion of IL-6.
Thy0517 with down-regulated HMGB1 was co-cultured with PBMC; the percentage of Th17 cells in PBMC was lower than the control; the percentage of Treg cells was higher than the control.