Introduction
People change their hair colours with dyes to enhance their beauty and to keep up with fashion. Increased use of hair dyes regardless of the economic and educational situation commonly causes side effects. Commonly used permanent hair dyes are produced as a result of an oxidative process involving arylamines. Oxidative hair dyes have been reported to be associated with contact dermatitis and hair loss [1, 2]. Some constituents of hair dyes have been shown to be carcinogenic in animals [3]. In addition, some constituents were shown to cause dermatitis and hair loss through oxidative stress and epidermal inflammation [4].
Aim
Permanent hair dye, oxidant cream, and henna are used frequently to change hair colour. The aim of this study was to investigate the effects of these colour changing agents on oxidative status in serum and liver specimens of rats.
Material and methods
For this study, approval was obtained from the Animal Trials Local Ethics Committee of Sutcu Imam University (session no. 2018/01, decision no. 01, approval date: 13.02.18). Specific national law on the protection of animals was observed. Twenty four female Wistar albino rats (body weight 200–300 g) were randomly selected for this study and four groups were formed as the control (n = 6), the hair dye (n = 6), the oxidant cream (n = 6), and the henna (n = 6) groups. A 2-week period was given before the experiments to allow the animals get familiar with the environment. Animals were maintained at 22°C ambient temperature and 60 ±5% humidity with access to food and water in a 12-hour light/12-hour dark cycle.
Preparation of the hair dyes
Hair dye (Koleston brand/red colour), oxidant cream (Mosse brand, containing 6% hydrogen peroxide), and henna (Camel brand from India) were purchased at the supermarket. The hair dye was prepared by mixing dye mixture with an oxidant cream included in the product box in a container and made ready for use. The oxidant cream was ready for use when it was purchased. Powdered henna was prepared by homogeneously mixing with a small amount of tap water in a container. The products that changed hair colour were applied to rats using the method they were applied to humans. First, rats were anesthetized intraperitoneally with 80 mg/kg ketamine and 10 mg/kg xylazine. The hair colour modifying products were applied in an area of about 4 × 4 cm on the back of the rats, and thicknesses of 0.3–0.5 cm with hair dye and oxidant cream and 0.5–1 cm with henna were achieved.
Collection of tissue and blood samples
The rats were washed with 25°C tap water for 50 min after application as described in the hair dye and oxidative cream ingredients. After washing, the rats were dried and body heat was stabilized by the radiant heater. An hour after washing, rats in groups which were treated with hair dye and oxidant cream were sacrificed by cervical dislocation. Rats in the henna group were sacrificed by cervical dislocation 7 h after the application. The rats in this group were not washed due to spontaneous separation of henna from the skin over time. After sacrificing, the areas where the hair colour changing products were applied were shaved with a razor blade. Approximately 1 cm skin was excised from each rat. Blood samples were taken by cardiac puncture and centrifuged. Liver tissues were cut to assess oxidative stress parameters. All tissue and blood samples were kept overnight at –80°C for biochemical evaluations.
Biochemical analysis
On the day of analysis, liver samples were homogenized in ice-cold 0.15 M KCl (10%, w/v). The resulting homogenates were centrifuged at 600xG for 10 min at 4°C to remove the crude fractions. Subsequently, the supernatants were centrifuged at 10,000xG for 20 min to obtain the postmitochondrial fraction. Superoxide dismutase (SOD), nitric oxide (NO) and glutathione peroxidase (GSH-Px) activities were determined in the postmitochondrial fraction and serum obtained from the liver. Postmitochondrial fraction protein determinations were performed using bicinonic acid. Malondialdehyde (MDA) levels were determined in serum and homogenates obtained from the liver. Serum and tissue MDA levels were measured with the method defined by Ohkawa et al. [5] after minor modifications. SOD activity was measured by the method described by Beyer and Fridovich [6]. In this method, xanthine and xanthine oxidase are used to form superoxide radicals which react with 2-(4-iodophenyl)-3- (4-nitro phenols phenyl tetrazolium chloride) to form red formazan dye. SOD activity was then measured by the degree of inhibition of this reaction. The Beutler method was used for GSH-Px activity measurement. GSH-Px catalyses the oxidation of reduced glutathione (GSH) to oxidized glutathione (GSH) via H2O2. GSH-Px activity was determined by the spectrophotometric absorbance difference at 340 nm due to oxidation reaction of NADPH to NADP. Since skin samples were firm, they could not be homogenized and biochemical analysis could not be done.
Statistical analysis
SPSS 16.0 software was used for statistical analysis. Nonparametric Kruskal-Wallis test and Mann-Whitney U test were used to assess the differences between continuous data. Box plots were used for group comparisons. The Pearson correlation test was used to evaluate the relationship between two continuous variables. The statistical significance level was accepted as p < 0.05.
Results
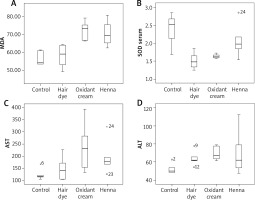
The Kruskal-Wallis test showed significant differences in serum SOD, aspartate aminotranspherase (AST), alanine aminotranspherase (ALT), and liver MDA values among the study groups. Subgroup averages and Kruskal-Wallis test results of oxidative markers and AST, ALT in serum samples of rats are given in Table 1. Subgroup averages and Kruskal-Wallis test results of oxidative markers in liver samples of rats are given in Table 2. Comparison of parameters which show a statistically significant difference with Mann-Whitney U test between binary groups is given in Table 3. There was no significant correlation between serum SOD and serum GPX and between serum SOD and liver MDA values (p and r values are 0.964, 0.010; 0.666, 0.093, respectively). However, there were statistically significant positive correlations between liver MDA and ALT-AST values (p and r values: 0.001, 0.616; 0.023, 0.463, respectively) (Figure 1).
Table 1
Subgroup averages and Kruskal-Wallis test results of oxidative markers and AST, ALT in serum samples of rats
Table 2
Subgroup averages and Kruskal-Wallis test results of oxidative markers in liver samples of rats
Table 3
Comparison of parameters which show a statistically significant difference with Mann-Whitney U test between binary groups
Discussion
In biological systems, electron accepting molecules are known as free radicals. Free radicals are called oxidants when they are active oxygen derivatives. Oxidants lead to damage by adversely affecting cell membrane, genetic material or various enzymatic events when antioxidant defence capacity is overwhelmed [7]. An organism may also produce free radicals due to xenobiotics and normal metabolic activities [8]. Human skin has the capacity to metabolize xenobiotics. However, enzyme levels involved in the metabolism of xenobiotics in the skin are 4–10 times less than those found in the liver [9]. It is known that many personal care products applied on the skin or chemicals that come in contact with the skin can be absorbed through the skin. For this reason, the skin area to which the product is applied, blood, and liver tissues due to percutaneous absorption are the target structures of the chemicals in the product [10]. It is known that 50–80% of women in the world use hair dyes at least once in their lives [11].
In this study, it was determined that hair dye, oxidant cream, and henna have an oxidative effect by decreasing the serum SOD value or increasing the liver MDA value. In addition, three products that changed hair colour were observed to have hepatotoxic effects by increasing AST and/or ALT levels. Surprisingly, in intergroup comparisons, it was found that oxidative effects and liver toxicities of the oxidant cream and henna were similar. The oxidant cream was found to be more oxidative and more toxic to the liver when compared to hair dye.
SOD catalyses conversion of hydrogen peroxide to superoxide. GPX removes hydrogen peroxide from the tissues. SOD and GPX inhibit the accumulation of free radicals and the onset of lipid peroxidation. In this study, serum SOD levels were found to be low in hair dye and oxidant cream groups, which was possibly associated with increased oxidant burden. SOD catalyses conversion of hydrogen peroxide to superoxide. MDA is formed by the oxidation of fatty acids in the cell membrane and is the most important indicator of lipid peroxidation. In this study, the liver MDA value was found to be high in the oxidant cream and the henna groups. Oxidative stress refers to a shift in prooxidant-antioxidant balance towards the prooxidant side leading to cellular damage [7]. In the tissue culture model developed by Baker and Staecker [12], it was observed that repeated oxidative stress significantly increased hair cell death. In another study conducted by Jeong et al. [13], rat skin was bleached with hydrogen peroxide. Morphologic examination of the skin revealed that the extracellular matrix was disrupted, the epidermis was thinned, and subepidermal bullae were formed. Because the skin samples obtained in this study could not be homogenized, the oxidative status in the skin could not be assessed. It has been shown that hair dyes cause irritation in the ear, neck, and shoulder skin and that absorption of toxic substances they include cause hair loss and some cancers. Hydrogen peroxide has been shown to cause oxidative stress and cytotoxicity, suppressing hair growth [4, 10]. In their study, Zanoni et al. [14] reported that paraphenylenediamine (PPD) and hydrogen peroxide, included in the hair dye, cause oxidative stress and subsequent DNA damage by increasing the formation of reactive oxygen species in human keratinocytes. Although the hair dye and the oxidant cream evaluated in this study included the same amount of hydrogen peroxide, the oxidant cream was more oxidative and hepatotoxic compared with the hair dye although the difference was not statistically significant. We think that this may be due to dilution of the oxidant cream after mixing with hair dye mixture during preparation of hair dye. Henna (Lawsonia inermis), a flowering plant, is grown in North Africa, India, Pakistan, and Ceylon. It has been reported that henna is used in the treatment of diseases such as eczema, burns, headache, scar, diarrhoea, leprosy, and fungus [15]. Numerous studies have been conducted on the efficacy and reliability of henna and contradictory results have been reported. In some studies emphasizing the positive aspects of henna, it has been shown that henna have antioxidant, antimicrobial and anti-inflammatory effects and does not cause significant genotoxicity [16, 17]. In one study, extracts of Lawsonia inermis have been shown to improve plasmodium infection in mice by enhancing the endogenous antioxidant system and by suppressing oxidative damage [18]. Petzel-Witt et al. applied hydrogen peroxide, henna, permanent dye, and bleach to natural hair samples. Persistent hair dye and bleach were found to be oxidative, whereas henna was found to have no effect on the oxidative status of hair [19]. Various studies reported that henna has hepatoprotective effects resulting from its antioxidant properties [20, 21]. On the other hand, the FDA has not approved the direct application of henna to the skin [14]. A study by McMillan et al. [22] demonstrated that henna caused haemolysis in rats associated with oxidative damage. A study by Sauriasari et al. [23] showed that two of the marketed henna samples showed cytotoxic effects whereas natural henna leaves did not have such effects. The resulting oxidative stress was associated with cytotoxic damage. The use of henna samples obtained by natural or unnatural pathways may be the reason for these different results in the literature. In this study, marketed henna samples were used and compared with the control group, hepatotoxic and oxidative effects of henna were observed.
Conclusions
Women and men with various sociocultural and economic levels are increasingly using products that change hair colour. Although permanent hair dyes, oxidant creams, and henna are frequently used, reliability is still a matter of debate. In this study, which is an “animal model study”, it was found that all three products had oxidative and hepatotoxic effects beyond the changing hair and skin colour. Of course, these results cannot be directly transferred to humans and further investigations are needed on humans. More information on these products will prevent potential damage.