Introduction
Airborne fine particulate matter (FPM), produced by air dust pollution, combustion activities, etc., severely threatens human health. Ambient particular matter pollution ranked fifth among the global death risk factors in 2015 [1]. Exposure to FPM increases the rate of hospitalization for respiratory and cardiovascular diseases [2]. Maternal exposure to ambient particulate matter affects the fetus’s development [3]. Asthma, bronchitis, coronary artery disease, atherosclerosis, and congestive heart failure are also related to FPM. FPM-triggered inflammation is implicated in the pathogenesis of these diseases [4].
Macrophages play a significant role in FPM-induced systemic inflammation. Macrophages can be polarized into M1 phenotype and M2 phenotype [5]. M1-type macro- phages are triggered by lipopolysaccharide or Th1 cytokines and produce pro-inflammatory cytokines. Th2 cytokines activate M2-type macrophages, which produce anti-inflammatory cytokines. M1-type macrophages are pro-inflammatory and promote chronic inflammatory con- ditions. M2-type macrophages are anti-inflammatory and capable of repairing tissues. In infected tissues, macro- phages firstly polarize to M1-phenotype to remove pathogens and then convert into the M2 phenotype to repair damaged tissue. Macrophage polarization determines the fate of tissues [6]. The balance of M1- and M2-type macrophages guarantees tissue health. The imbalance of macrophage phenotypes facilitates the progression of related diseases. Unbalanced M1-type polarization contributes to the uncontrolled inflammatory immune response in autoimmune diseases [5]. FPM exposure aggravates autoimmune diseases, which are associated with M1-type/M2-type imbalance [7, 8]. M1-type polarization inhibition protects against particulate matter induced injury [9]. Unbalanced M2-type polarization promotes angiogenesis, organ fibrosis, tumor growth, and infection [10]. Exploring the mechanism of macrophage polarization helps to manipulate the balance of M1- and M2-type macrophages.
Fine particulate matter can affect macrophage polarization directly [11] or indirectly [12]. The effects of particulate matter on macrophage polarization are inconsistent in previous studies. FPM was shown to increase the pro- inflammatory cytokines of macrophages and enhance lipo- polysaccharide (LPS)-induced M1-type polarization after in vitro stimulation [11]. Pro-inflammatory cytokines, which promote M1-type polarization, were observed to be increased in a dose-dependent manner after exposure to residential FPM with an aerodynamic diameter ≤ 2.5 µM (PM2.5) [13]. However, M1 phenotype-related inflammatory genes were dramatically decreased, and M2 phenotype-related anti-inflammatory genes were progressively activated after exposure to cigarette smoke particulates [14]. In an ovalbumin-induced asthma mouse model, particulate matter was shown to aggravate M2-type polarization [15, 16]. These contradictory results may be the product of the complexity of particulate matter [17]. Exposure duration is likewise a determinant of macrophage phenotype. An inflammatory marker, CRP, increased in participants with metabolic syndrome after exposure to PM2.5, and the increasing response was more pronounced in the long-term (30-60 days) exposure group [18]. The content of the M2-type macrophage marker CD206 in alveolar macrophages decreased after four days of biomass fuel smoke exposure but increased after six months of exposure [19]. The dynamic change of macrophage phenotype is involved in the pathogenesis of FPM-related diseases. FPM accelerates lung injury by promoting M1-type polarization [20]. Both M1-type and M2-type macrophages were progressively activated with smoking [21] and correlated with the pulmonary collagen volume fraction [22]. Chronic exposure to carbon black ultrafine particles promotes an immunosuppressive microenvironment through metabolically rewiring lung macrophage [23]. Nevertheless, the mechanism of macrophage dynamic phenotype change remains unclear.
In this study, concentrated ambient FPM was utilized to stimulate macrophages in vivo. Macrophage polarization was measured after different exposure duration. It demonstrated that the macrophage phenotype changes with the time of FPM stimulation. Short-term FPM exposure in vivo or acute stimulation in vitro promotes M1-type polarization. With the increase of exposure duration, macrophages gradually transformed to M2-type polarization. This study serves as a theoretical basis for revealing the mechanism of disease occurrence under the FPM environment.
Material and methods
Mice
Six- to eight-week-old C57BL/6 mice were purchased from SPF (Beijing) Biotechnology Co. (Beijing, China). All mice were specific pathogen-free (SPF). The ethics committee of Strategic Support Force Medical Center approved all procedures.
Peritoneal macrophages isolation and culture
The mice were sacrificed and soaked in 75% alcohol for 1-2 minutes. The abdominal skin was cut to expose the abdominal muscle layer. Five-milliliter RPMI1640 culture medium (Cytiva, USA) was injected into the abdominal cavity. The abdomen was gently rubbed with a cotton ball for 1-2 minutes, and then the cell suspension was collected and transferred into a centrifuge tube. After centrifugation at 1000 rpm for 5 minutes, the supernatant was discarded. The cell precipitation was washed with RPMI1640 medium and re-suspended with the culture medium (RPMI1640 medium containing 10% calf serum (Hyclone, USA) and 1% penicillin-streptomycin solution (Hyclone, USA)). The cells with a cell density of 2 × 106 cells/ml were transferred to a 6-well flat bottom culture plate. Unattached cells were discarded after incubation in a 5% CO2 incubator for 4 hours. The adherent cells were monolayer peritoneal macrophages.
FPM treatment in vitro
The peritoneal macrophages were treated with FPM (Sigma-Aldrich, Germany) suspension of 0 µg/ml, 50 µg/ml, and 100 µg/ml for 6 hours. The purchased FPM is urban atmosphere particulate matter. The stimulation dose corresponded to the existing literature [24-26]. Cell morphology and cell number were observed with a light microscope. Expression of different genes was detected with quantitative real-time PCR (qRT-PCR) and western blotting.
FPM treatment in vivo
Six- to eight-week-old C57BL/6 mice were divided into two groups: the control group and the exposure group. Data from the 2021 Beijing Ecological Environment Status Bulletin showed that the annual mean concentration of PM2.5 was 33 µg/m3. The well-defined hazardous concentration of PM2.5 is 250.5 µg/m3. Thus, ten times ambient FPM concentration was chosen. Mice in the exposure group were raised in the exposure cabin, concentrating the ambient FPM ten times. Mice in the control group were raised in the SPF mouse facility. The mice were sacrificed after exposure to concentrated ambient FPM for 7 days, 14 days, and 3 months. No accidental death occurred during the experiment.
Total RNA extraction and reverse transcription
Peritoneal macrophages were lysed with 500 µl of Trizol reagent (Invitrogen, USA). One hundred microliters of chloroform was added to the lysate and mixed thoroughly. The mixture was centrifuged at 12,000 rpm for 10 minutes. The supernatant was isolated, and an equal volume isopropanol was added. The mixture was centrifuged at 12,000 rpm for 10 minutes. The supernatant was discarded, and the precipitate was washed with 75% alcohol twice. The precipitate was dried and dissolved with 30 µl of RNase-free water. The concentration of total RNA was measured with the NanoDrop ND-1000 spectrophotometer (Nanodrop, USA).
qRT-PCR
The total RNA was reverse transcribed with the Tiangen Fastking cDNA synthesis kit (Tiangen, China) according to the manufacturer’s instructions. Four pairs of primers were synthesized by Qingke Biological Company, and the sequences of primers are shown in Table 1. Real-time PCR was performed using the Tiangen Real-time fluorescence quantitative PCR kit (Tiangen, China) according to the manufacturer’s instructions. The reactions were run on a LightCycler 480 real-time detection system (Roche, Switzerland). The reactions were performed as follows: 95oC for 5 minutes for the initial denaturation and then 40 cycles of 95oC for 15 s, 60oC for 40 s, and the melt curve was analyzed from 60oC to 95oC at 1oC increments. The expression of GAPDH was used as an internal reference. The relative expression levels of genes were calculated by 2-ΔCt.
Table 1
Primers used in qRT-PCR
Flow cytometry
The culture medium was discarded, and the cells were washed with phosphate buffered saline (PBS) (Hyclone, USA) three times. The cells were harvested and washed twice with the washing liquid (PBS containing 1% bovine serum albumin [BSA]). The cells were re-suspended with the washing liquid and divided into two tubes. The PE/Cy7 anti-mouse CD206 antibody (BioLegend, USA) and Rat IgG (ZSGB-Bio, China) were added, respectively. The mixtures were incubated at 4oC for 30 minutes. The reaction tube was mixed upside down every 10 minutes during incubation. The mixtures were centrifuged at 1000 rpm for 5 minutes, and the supernatant was discarded. The cells were washed with PBS three times and re-suspended with 4% paraformaldehyde. The cells were detected with flow cytometry (BD, USA).
Results
In vitro FPM stimulation did not affect the number of macrophages
Macrophages are the first line to clear FPM. To study the effects of FPM on macrophages, we isolated and cultured peritoneal macrophages of mice. The mature macrophage ratio in all cells was detected with flow cytometry, and the percentage of mature macrophages was 80.49% (Supplementary Fig. 1), indicating that most of the isolated cells were macrophages. Peritoneal macrophages were treated with 0 µg/ml, 50 µg/ml, and 100 µg/ml of FPM in vitro. The cell number and morphology were observed through the light microscope before and after stimulation. The results showed that mouse peritoneal macrophages grew well before FPM stimulation (Fig. 1). No apparent changes in cell number or cell morphology among different treatments were found after 6 hours of stimulation with varying concentrations of FPM (Fig. 1).
Fig. 1A-H
Mouse peritoneal macrophages before and after in vitro fine particulate matter (FPM) stimulation. Mouse peritoneal macrophages were isolated and cultured for 20 h, and then the macrophages were stimulated with different concentrations of FPM (0 μg/ml, 50 μg/ml, 100 μg/ml). Images of macrophages were taken after 6 hours of stimulation. Pre represents pre-treatment. 200× indicates a magnification of 200. 400× indicates a magnification of 400. The scale in the picture is 100 μM
Acute FPM stimulation in vitro promotes M1-type polarization
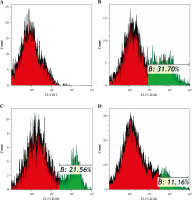
Fine particulate matter can affect macrophage polarization directly. To investigate the macrophage phenotype change challenged with FPM, we detected the expression levels of macrophage phenotype markers. NLRP3 inflammasome mediates M1-type polarization, and the production of NLRP3 increased in M1-type macrophages [27]. NLRP3 deficiency attenuated FPM-induced lung injury [28]. NOS2 is a marker of M1-type macrophage, and ARG1 is a marker of M2-type macrophage [29]. Thus, we detected the expression of NLRP3, NOS2, and ARG1 with qRT-PCR. The results showed that the expression of NLRP3 and NOS2 increased, and the expression of ARG1 decreased with increasing FPM concentration (Fig. 2A-C). To further verify the results, we detected the expression of NLRP3 with western blotting. The results showed that the expression of NLRP3 increased with the rising FPM concentration, consistent with the result of qRT-PCR. CD206 is another marker of M2-type macrophages [5]. Flow cytometric analysis was performed to detect the proportion of CD206-positive (CD206+) macrophages. It demonstrated that the proportion of CD206+ cells decreased with increasing FPM concentration. After stimulation with 0 µg/ml, 50 µg/ml, and 100 µg/ml FPM, the percentages of CD206+ cells were 31.7%, 21.56%, and 11.16%, respectively (Fig. 3). These results suggest that in vitro FPM stimulation enhances M1-type polarization and inhibits M2-type polarization in a dose-dependent manner. Acute FPM stimulation promotes the pro-inflammatory function of macrophages.
Fig. 2
Detection of M1-phenotype marker and M2-phenotype marker expression in peritoneal macrophages after acute fine particulate matter (FPM) stimulation in vitro. A-C) Measurement of the relative expression of ARG1, NOS2, and NLRP3 with qRT-PCR. D) Western blotting analysis of NLRP3. GAPDH was used as an internal reference
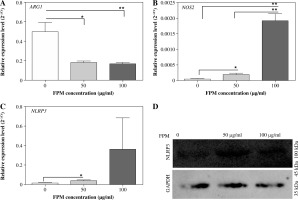
Fig. 3
Flow cytometric analysis of CD206+ macrophages after acute fine particulate matter (FPM) stimulation in vitro. A) Unlabeled negative cells. B-D) Cells labeled with PE/Cy7 anti-mouse CD206 antibody. Green areas are CD206 expression region. B) Macrophage stimulated with 0 μg/ml FPM, in which the CD206+ macrophage ratio is 31.7%. C) Macrophage stimulated with 50 μg/ml FPM, in which the CD206+ macrophage ratio is 21.56%. D) Macrophage stimulated with 100 μg/ml FPM, in which the CD206+ macrophage ratio is 11.16%. The Y-axis is fluorescence intensity, and the X-axis is the relative cell number
Macrophage phenotype changed with FPM exposure duration
To further study the effect of FPM on macrophage polarization, we conducted in vivo FPM challenge. Six- to eight-week-old mice were randomly divided into two groups: the control group and the exposure group. Mice were sacrificed, and peritoneal macrophages were isolated after exposure of different duration. The expression of NLRP3, NOS2, and ARG1 in peritoneal macrophages was detected by qRT-PCR. The results showed that the expression of NLRP3, NOS2, and ARG1 changed with the FPM exposure time. The expression levels of NLRP3 and NOS2 were significantly increased. The expression of ARG1 was significantly decreased after 7 days of exposure to concentrated ambient FPM (Fig. 4A). NLRP3, NOS2, and ARG1 were up-regulated after 14 days of FPM exposure (Fig. 4B). The expression of NLRP3 decreased. The expression of ARG1 increased significantly after 3 months of FPM exposure (Fig. 4C). The expression of NOS2 was not detected after 3 months of FPM exposure. These results implied that FPM exposure time affects peritoneal macrophage polarization.
Fig. 4
Detection of M1-phenotype marker and M2-phenotype marker expression in peritoneal macrophages after different PM2.5 exposure duration in vivo. A) Relative expression level of ARG1, NLRP3, and NOS2 after 7 days of FPM exposure. B) Relative expression level of ARG1, NLRP3, and NOS2 after 14 days of FPM exposure. C) Relative expression level of ARG1 and NLRP3 after 3 months of FPM exposure

Discussion
The adverse impacts of atmospheric FPM on health have become an important public health issue. Exposure to FPM not only increases the risk of respiratory and cardiovascular diseases but also affects the central nervous system and brain health. FPM exposure can cause systemic inflammation [30]. Macrophages, the sentinel of innate immunity, play an essential role in this process. Conversion between M1- and M2-type macrophages is a crucial determinant of the inflammatory state. In this research, we studied the effect of FPM exposure duration on peritoneal macrophage polarization.
Direct FPM stimulation of peritoneal macrophage was conducted in this study. In agreement with the published studies, we found that acute FPM stimulation promoted M1-type polarization and inhibited M2-type polarization [11]. It is well established that FPM-triggered hyper-inflammation is a crucial factor in the pathogenesis of FPM-associated diseases [4, 31]. Particulate matter exposure causes persistent lung inflammation [32]. Concentrated ambient FPM exposure causes systemic cytokine activation [33]. Macrophages are essential in processing inhaled particulate matter, and macrophage polarization plays an essential role in FPM-induced hyper-inflammation. Inhibition of the inflammatory response in macrophages protects against adverse effects caused by particulate matter exposure [34]. Nevertheless, this cannot explain the immune suppression caused by particulate matter [35, 36]. Here, we found that the macrophage phenotype is dynamic, varying with FPM exposure duration. Short-term FPM exposure promoted M1-type polarization, and long-term FPM exposure promoted M2-type polarization. Our findings suggest that the anti-inflammation caused by long-term FPM exposure is also implicated in the pathogenesis of FPM-related disorders. This suggestion could also be verified in infectious diseases. FPM exposure time was found to have an impact on pathogen resistance. Short-term FPM exposure increased the survival rate of mice infected with influenza A, while long-term FPM exposure lowered the survival rate [37]. M2-type polarization triggered by long-term FPM exposure accelerates the inflammatory state change and affects the pathogen clearance of the immune system. Thus, we hypothesized that immune regulation is more critical than immune inhibition in FPM-caused diseases.
Dynamic phenotype change of alveolar macrophage has previously been observed in chronic biomass ambient particulate matter exposure. M1-type macrophages were converted to M2-type macrophages after six months of biomass fuel smoke exposure [19]. Alveolar macrophages are the first line to clear up FPM. However, the systemic immune response change after FPM exposure is not clear. In this study, we investigated the effect of FPM on the peritoneal macrophages, which have no direct contact with FPM and partly reflect the systemic immune response to FPM. Peritoneal macrophages changed from M1-type to M2-type with the increase of exposure duration. It may indicate that the systemic immune response changed from immune enhancement into immune suppression. A systemic immune response change affects many diseases. Tumorigenesis and metastasis were aggravated by FPM exposure through regulating macrophages [23, 38]. Systemic immune response conversion may contribute to the pathogenesis of these diseases.
Our results provided a different perspective on the pa- thogenesis of FPM-associated disorders. The dynamic phenotype change of macrophages with exposure duration also suggests that the reversibility of macrophages has important therapeutic value, especially in diseases caused by the imbalance of M1- and M2-type macrophages. How to regulate the reversibility of macrophages and maintain M1- and M2-type macrophage balance is worthy of further exploration.


