Introduction
Homeobox genes are a group of genes containing the homeobox DNA sequences that encode homeodomains [1]. The homeodomain consists of four α-helices, a helix-turn-helix motif, and a flexible N-terminal arm that regulate the expression of target genes through the interaction between homologous molecules. This is achieved through the binding of the helix-turn-helix structure to target genes [2]. In the human genome, there are 300 homeobox loci, including 235 functional genes and 65 pseudogenes. These genes are divided into 102 gene families that are divided into 11 gene classes, widely recognized as ANTP, PRD, LIM, POU, HNF, SINE, TALE, CUT, PROS, ZF, and CERS [3]. Among them, ANTP is the largest gene class, hosting the widely known HOX genes [4]. The homeobox gene family has highly conserved DNA-binding domains and plays an important role in embryonic development, including the morphogenesis and differentiation of various tissues and organs [5].
Homeobox containing 1 (HMBOX1), a transcription factor with transcriptional inhibitory activity, was isolated from a cDNA library of the human pancreas in 2006 and belongs to the HNF gene class of the homeobox gene family. The full length of the HMBOX1 gene is 1263 bp and it encodes a protein containing 420 amino acid residues. It contains a highly atypical homeo domain at the N-terminus and is homologous to hepatocyte nuclear factor 1 (HNF1). HMBOX1b, a novel HMBOX1 splicing variant located in both the nucleus and cytoplasm, might function differently from HMBOX1 [6].
A database search revealed that HMBOX1 was widely expressed in various human tissues, but its expression level was uneven. The protein expression level was relatively high in the brain, adrenal gland, testis, and skin. It is expressed with low specificity in both tumor and immune cells and is mainly localized to the nucleoplasm (these data come from Human Protein Atlas). HMBOX1 also acts as a direct telomere repeat-binding protein, known as homeobox telomere binding protein 1 (HOT1). In recent years, in addition to regulation of differentiation and development, its role in tumors, inflammation and other diseases has gradually attracted attention. In the current review, we would like to provide the emerging evidence for the relevance of HMBOX1 in cell biological function and the immunoregulation process and discuss the potential of HMBOX1 as a target for immune-related diseases.
HMBOX1 and cell differentiation
The homeobox gene has been shown to be involved in regulating the differentiation of various tissues and cells [7-11], including HOXC8 suppression in osteo-/dentinogenic differentiation [7], NKX2-3 and NKX2-4 in acute myeloid leukemia subtype formation [8], and Six3 in medium spiny neuron differentiation [10] and caudal repression of intestinal stem cell differentiation [11]. As a member of the homeobox family, HMBOX1 also plays an important role in cell differentiation and functional maintenance. Previous research confirmed that HMBOX1 plays an important role in regulating the differentiation of embryonic and bone marrow stromal stem cells (BMSCs) into vascular endothelial cells (VECs) [12, 13]. By exploring this mechanism, we found that activation of the HMBOX1/CD163/FGF-2 signaling pathway is involved in regulating the differentiation of BMSCs into vascular endothelial-like cells [14]. Suppressing the expression of IP-10 and increasing that of Ets1 were also considered as other mechanisms for the differentiation of BMSCs into VECs [12, 13]. Moreover, HMBOX1 is highly expressed in normal human VECs and is essential for their survival [15]. By interacting with metallothionein 2A, it can increase the level of free Zn2+ in VECs [16], further promoting human VEC autophagy by inhibiting mTOR signaling and cell apoptosis by affecting the level of cleaved caspase-3 [17, 18]. Furthermore, it was proven that heterogeneous nuclear ribonucleoprotein E1 (hnRNP-E1) could directly bind to the 5-UTR region of HMBOX1 to promote its expression, which is essential for maintaining endothelial function [18]. In summary, HMBOX1 plays an important role in the differentiation and function of endothelial cells; however, most of the current studies lack direct evidence on the downstream genes regulated by HMBOX1, and more research is needed to discover the further mechanisms of its regulating effect on endothelial cell behavior.
HMBOX1 and immune regulation
The homeobox gene, especially the NKL gene subclass, has been widely confirmed to regulate the differentiation and development of immune cells including T cells, B cells, natural killer (NK) cells, and dendritic cells, and an imbalance in its regulatory function can induce hematological malignancies [19-22]. However, the role of homeobox genes in the activation and function of the immune system and immune cells remains unclear.
Recent studies have shown that HMBOX1 expression is negatively correlated with NK cell activation and function. HMBOX1 is highly expressed in resting NK cells, inhibits CD107a and cytolysin expression levels and suppresses the NKG2D/DAP10 signaling pathway, thus significantly inhibiting NK cell activity [23]. Activated NK cells show a significant decrease in HMBOX1 levels, accompanied by increased interferon γ (IFN-γ) levels. Further research confirmed that HMBOX1 can negatively regulate IFN-γ expression by inhibiting the transcriptional activity of the IFN-γ promoter [24]. However, these studies lack the support of in vivo experimental data and are limited to in vitro cellular level studies. HMBOX1 can affect tumor progression by regulating NK cell function. miR-30c-1 has been reported to increase the killing ability of NK cells towards human hepatoma cells by targeting the expression of HMBOX1 in NK cells [25]. However, most current studies focus on NK cells, and these studies are relatively limited. The immune regulatory role of HMBOX1 after it has altered the activation and function of NK cells is still little studied. In view of the effect of HMBOX1 on cell differentiation and development, more studies are needed to evaluate both the differentiation and development of immune cells and the maintenance of immune cell function.
HMBOX1 and inflammation
Homeobox genes are involved in the regulation of various inflammatory responses, including inhibition and facilitation [26-29]. For instance, activation of endothelial BMP4-HOXB9-TNF signaling is involved in the formation of arterial inflammation [30]. SIX1 reduces inflammation and rheumatoid arthritis symptoms by silencing the Myd88-dependent TLR1/2 signaling pathway in synovial fibroblasts [31].
At present, HMBOX1 has been confirmed to inhibit inflammation by targeting NF-κB, MAPK, and other forms of inflammatory signaling. HMBOX1 mitigates lipopolysaccharide (LPS)-induced human periodontal ligament stem cell injury by reducing CXCL10 expression through impeding the activation of the NF-κB signaling pathway [32]. In an endothelial model of atherosclerosis, HMBOX1 expression inhibited the endothelial inflammatory response induced by MAPK, the NF-κB pathway, and ROS activation [33]. HMBOX1 is involved in reversing miR-885-5p-induced elevation of interleukin (IL)-1β, IL-18, NLRP3, caspase-1 and GSDMD-N in human myocardial AC16 cells treated with sepsis-exos through the mechanism of NF-κB dependence [34]. These results suggest that HMBOX1 is a new diagnostic marker and therapeutic target for atherosclerosis. Currently, in the reports regarding inhibition of the inflammatory response, HMBOX1 is mainly believed to play a role by inhibiting the NF-κB signaling pathway, but whether HMBOX1 can target the key factors in the NF-κB signaling pathway has not been proven. Studies on the regulatory mechanisms between HMBOX1 and other pathways (such as MAPK) are still insufficient; the previous studies were limited to the observation of changes in the expression of other molecules after the intervention altering HMBOX1 expression.
Immune cells play an important role in the occurrence and progression of inflammation; however, there are few studies on whether HMBOX1 participates in the regulation of inflammation by regulating the activation of immune cells and the release of inflammatory factors. Our previous study demonstrated that HMBOX1 can negatively regulate the NF-κB signaling pathway to weaken the inflammatory response in hepatocytes. Meanwhile, HMBOX1 inhibited macrophage and neutrophil infiltration into liver tissue by downregulating NF-κB/CCL2 signaling. HMBOX1 also affects macrophage activation and cytokine release. All of them synergistically alleviated LPS/D-GalN-induced liver injury [35]. The effect of hepatocyte HMBOX1 on macrophage function is currently under investigation, and further research in this area is needed.
HMBOX1 and tumor progression
An increasing number of studies have shown that homeobox genes are abnormally expressed in the process of tumorigenesis. This suggests that the homeobox gene family not only regulates cell differentiation and development, but also plays an important role in tumor progression [5, 36]. For example, overexpression of HOXC6 enhances BCL-2 mediated anti-apoptosis and promotes cervical cancer cell proliferation [37]. In colon cancer, HOXB13 can inhibit the proliferation of tumor cells by inhibiting the expression of c-Myc and inducing apoptosis of tumor cells, thus inhibiting tumor progression [38]. Interestingly, in gastric cancer, HOXB13 can promote the proliferation, invasion, and migration of tumor cells by activating PI3K/AKT/mTOR signaling [39]. Different homeobox genes can, therefore, regulate tumor progression through multiple mechanisms, and the same gene may play opposing regulatory roles in different tumors, suggesting that homeobox genes are functionally diverse in tumors.
As a member of the homeobox family, HMBOX1 is abnormally expressed in tumors, but its expression is inconsistent. For example, compared with that of adjacent normal tissues, HMBOX1 expression is low in liver cancer tissues, high in renal clear cell carcinoma tissues, and high in both pancreatic cancer and adjacent normal tissues [40].The expression and function of HMBOX1 in tumors depend on the corresponding tumor microenvironment. The difference in the expression levels also implies the diversity of the functions. In Table 1, we summarized relevant literature reports on the behavior of HMBOX1 in tumors.
Table 1
Summary of the behavior of HMBOX1 in tumors
| Author | Year | Tissue or cell type | Notes | Reference |
|---|---|---|---|---|
| Zhang P, et al. | 2017 | Glioma | Significantly correlated with WHO grade for glioma disease | [47] |
| Yu YL, et al. | 2018 | High-grade serous Ovarian cancer | Inhibits cell proliferation by promoting cell apoptosis | [41] |
| Zhao H, et al. | 2018 | Liver cancer | Promoting autophagy, inhibiting stemness and immune escape | [42] |
| Zhang M, et al. | 2019 | High-grade serous Ovarian cancer | Inhibits the proliferation, migration and invasion of ovarian cancer cell | [43] |
| Diao N, et al. | 2019 | Gastric cancer | Promotes cell proliferation and migration | [46] |
| Chen S, et al. | 2020 | Osteosarcoma | Inhibits osteosarcoma tumorigenesis | [45] |
| Zhou C, et al. | 2021 | Early-stage cervical cancer | Inhibits tumor immune escape | [44] |
| Dermawan JK, et al. | 2021 | Myxoid spindle cell neoplasm | ALK-HMBOX1-fusion occurred in myxoid spindle cell neoplasm | [50] |
| Dermawan JK, et al. | 2022 | Sarcoma | Neuregulin 1-HMBOX1-fusion occurred in high-grade spindle cell sarcomas | [49] |
HMBOX1 inhibits tumor progression
On one hand, the expression of HMBOX1 in tumor cells can directly inhibit the tumor through regulating cell apoptosis and autophagy. Compared with normal tissues and cells, the expression of HMBOX1 is decreased in ovarian cancer tissues and cell lines. Overexpression of HMBOX1 in ovarian cancer cell lines can downregulate the levels of anti-apoptotic proteins (Bcl-2 and Bcl-xL) and promote the expression of apoptotic regulatory proteins (Bad and Bax), caspase 3, and p53. All of them synergistically promote cell apoptosis and inhibit cell proliferation [41]. Our previous study found that the expression of HMBOX1 is decreased in hepatocellular carcinoma (HCC) and negatively correlated with the clinical staging. Consistent results were observed in HCC animal models and related cell lines. HMBOX1 expression in HCC cells has an inhibitory effect on tumors by promoting autophagy of tumor cells, inhibiting stemness formation, and increasing the sensitivity of tumor cells to NK cell killing [42]. In high-grade serous ovarian cancer patients, tRNA-derived fragments promote ovarian cancer cell proliferation, migration, and invasion by modulating HMBOX1 [43].
On the other hand, HMBOX1 can play an indirect tumor suppressive role by regulating the function of tumor-associated stromal cells and the tumor microenvironment. Recent studies have shown that the expression of HMBOX1-SOCS1 in tumor-associated lympho-endothelial cells can be downregulated by miRNA-1468-5p encapsulated in cervical cancer cell exosomes, thereby activating JAK2/STAT3 signaling, which upregulates the expression of lymphatic PD-L1 to inhibit the function of CD8+ T cells and help tumor immune escape [44]. These results suggest that HMBOX1 expression could inhibit tumors by inhibiting STAT3 signaling activation in lympho-endothelial cells. In addition, HMBOX1 plays a tumor-suppressing role by regulating the activation of tumor-related signaling pathways. As the downstream target of Wilms’ tumor 1-associated protein, HMBOX1 can inhibit the progression of osteosarcoma by inhibiting activation of the PI3K/AKT signaling pathway [45]. However, there are few studies on the regulatory function of HMBOX on tumor stromal cells, especially tumor-related immune cells, so further studies are required.
HMBOX1 promotes tumor progression
Similar to homeobox genes, such as HOXB13, HMBOX1 plays a double-sided role in tumors. However, the expression of HMBOX1 was significantly upregulated in gastric cancer and glioma and was correlated with tumor stage, lymph node metastasis and tumor microvasculogenesis [46, 47]. Zhou et al. confirmed that the c-Fos/miR-18 feedback loop promotes glioma proliferation via acting on HMBOX1 [48].
As a partner gene, HMBOX1 has also been observed to fuse with several key genes in tumor development. Neuregulin 1-HMBOX1 fusion has been reported in high-grade spindle cell sarcomas [49], as well as an aplastic lymphoma kinase–HMBOX1 fusion in myxoid spindle cell neoplasm [50]. However, limited details regarding these cases were obtained, and future studies are needed to better characterize the role of HMBOX1 fusion in tumorigenesis and development.
Although HMBOX1 has been observed to play different regulatory roles in different tumors, as a transcription suppressor, most studies contend that it mainly plays an inhibitory role in tumors. However, there are few relevant studies and the mechanism is not fully understood. Regardless, there is a need for the study of its role in various aspects, including tumor epigenetics, metabolism, and tumor microenvironment regulation.
HMBOX1 and telomere function
Some studies have shown that HMBOX1 expression is related to the maintenance of telomere function [51]. As a direct telomere repeat-binding protein, HMBOX1 is a positive regulator of telomerase recruitment and telomere elongation and acts via association with the active telomerase complex and promoting chromatin association with telomerase [52-54]. Knockdown of HMBOX1 reduces the expression of telomerase in cervical cancer cells, shortens the length of telomerase, reduces the expression of proteins related to DNA damage repair, promotes the sensitivity of tumor cells to radiotherapy, and increases radiation-induced apoptosis [55]. It is known that N6-adenosine (m6A) methylation modification regulates mRNA stability, splicing, transportation, and translation. Recent studies have shown that HMBOX1 is a target mRNA for m6A modification and contributes to maintaining telomere homeostasis [45, 56, 57]. Particularly in tumors, the m6A modification of HMBOX1 mRNA causes progressive telomere shortening and eventually generates genomic instability in cancer cells, which drives malignant progression [45, 56]. However, in chemical-induced male reproductive injury, chemicals enhanced the stability of HMBOX1 by inducing m6A modification and inhibited chemical-induced telomere dysfunction [57]. This is inconsistent with what is observed in tumors. This discrepancy may be caused by the different contexts. The function of m6A is context dependent; that is, the biological outcome is related to the cell types, external stimuli, subcellular localization of effectors and locations of m6A sites on mRNA. Therefore, when observing the regulatory effect of HMBOX1 on telomeres, it needs to be determined according to specific circumstances.
Conclusions and outlook
As a transcriptional suppressor, HMBOX1 has been studied less than other homeobox genes. Currently, research is mainly focused on its regulatory role in cell differentiation and development, inflammation, tumor development, and telomerase regulation (Fig. 1). The regulated molecules or pathways are summarized in Table 2. However, the specific mechanisms are still not fully understood and need to be explored in further studies. In addition, most of the current studies on the regulatory role of HMBOX1 as a transcription factor lack direct evidence and only observe the expression changes of downstream genes on the basis of the intervention on HMBOX1 expression. The research on targeting needs to be rich in research data. With the progress in experimental technology and techniques, higher requirements have been put forward for related research.
Table 2
Summary of HMBOX1 regulated molecules or pathways
| Author | Year | Tissue or cell type | Regulated molecules or pathways | HMBOX1 Function | Reference |
|---|---|---|---|---|---|
| Su L, et al. | 2010 | BMSCs | Downstream: IP-10, Ets-1 | Promote BMSCs’ differentiation to VECs | [12] |
| Han L, et al. | 2012 | ESCs | Downstream: FGF-2 | Promote ESCs’ differentiation to VECs | [13] |
| Lu W, et al. | 2016 | BMSCs | Downstream: CD163/FGF-2 | Promote BMSCs’ differentiation to vascular endothelial-like cells | [14] |
| Ma H , et al. | 2015 | HUVECs | Combine with: MT2A Downstream: mTOR, caspase-3 | Inhibit apoptosis and promote autophagy | [16] |
| Ma H, et al. | 2016 | HUVECs | Upstream: ANXA7/TGFB2-OT1/LARP1 | Essential for the survival of HUVECs | [15] |
| Meng N, et al. | 2019 | VECs | Upstream: hnRNP E1 Downstream: mTOR, caspase-3 | Inhibit apoptosis and promote autophagy | [18] |
| Wu L, et al. | 2011 | NK cells | Downstream: NKG2D/DAP10 | Negatively regulates NK cell functions | [23] |
| Wu L, et al. | 2011 | NK cells | Downstream: IFN-γ | Negatively regulates interferon γ production in NK cells | [24] |
| Gong J, et al. | 2012 | NK cells | Upstream: miR-30c-1* | Negatively regulates NK cell cytotoxicity | [25] |
| Zhao H, et al. | 2018 | Hepatocytes | Downstream: NF-κB/CCL2 | Negatively regulates infiltration of macrophages and neutrophils | [35] |
| Yuan HX, et al. | 2018 | EA.hy926 cells | Downstream: NF-κB and MAPK pathway | Inhibits LPS-induced inflammation | [33] |
| Tu GW, et al. | 2022 | Human myocardial cells (AC16) | Upstream: miR-885-5p | Inhibits the elevation of IL-1β, IL-18, NLRP3, caspase-1, and GSDMD-N in AC16 cells in an NF-κB dependent way | [34] |
| Nie M, et al. | 2022 | Human periodontal ligament stem cells | Downstream: NF-kB/CXCL10 | Attenuates LPS-induced periodontal ligament stem cell injury | [32] |
[i] BMSCs – bone marrow stromal cells, VECs – vascular endothelial cells, ESCs – embryonic stem cells, MT2A – metallothionein 2A, HUVECs – human umbilical vein endothelial cells, ANXA7 – annexin A7, TGFB2-OT1 – TGFB2 overlapping transcript 1, LARP1 – La-related protein 1, hnRNP E1 – heterogeneous nuclear ribonucleoprotein E1, IFN-γ – interferon γ
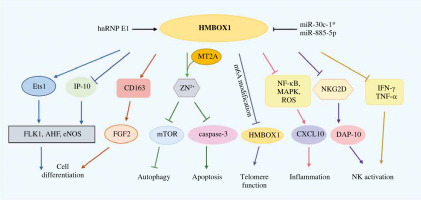
Fig. 1
HMBOX2 function summary diagram. Lines ending with arrows or bars indicate activating or inhibitory effects, respectively
MT2A – metallothionein 2A, hnRNP E1 – heterogeneous nuclear ribonucleoprotein E1, IFN-γ – interferon γ, TNF-α – tumor necrosis factor α, m6A – methylation on N6-adenosine, FGF-2 – fibroblast growth factor 2, Ets1 – ETS proto-oncogene 1
At present, HMBOX1 is believed to mainly play an anti-inflammatory role in inflammatory responses. However, whether, like other homeobox genes, it can function as a pro-inflammatory protein remains to be explored. Currently, HMBOX1 is considered to be a double-edged sword in tumor progression and may have different functions in different tumor cells, suggesting that tumor cell types should be considered when designing treatment regimens targeting HMBOX1. In addition, there are no small molecule inhibitors targeting HMBOX1, and the inhibition of HMBOX1 expression is mainly through gene level intervention. The tumor microenvironment, especially the immune-related microenvironment, plays an important role in the occurrence and development of malignant tumors. However, whether HMBOX1 is involved in regulating the formation of the microenvironment and the mechanism of this remain to be explored.
In general, the current research on the function of HMBOX1 is still in its infancy, and research on its role and mechanisms in various physiological and pathological conditions is insufficient and in need of further study. This, of course, suggests that there is still plenty of scope for researchers to study this molecule and that more attention should be paid to molecules in their infancy than to those that have been extensively studied.


