Introduction
Diabetes mellitus is a chronic metabolic disorder characterized by persistent hyperglycemia. Diabetes can be caused by impaired insulin secretion, resistance to the peripheral actions of insulin, or both [1]. It is classified as type 1 diabetes (T1D), type 2 diabetes, gestational diabetes mellitus (GDM), and a special type of diabetes caused by other factors [2]. This discussion focuses on T1D, which is characterized by an absolute lack of insulin due to the immune destruction of the islets [3].
In recent decades, the incidence of T1D has been growing globally [4]. Furthermore, at present, there is no cure for T1D; the cornerstone of therapy is diet and exercise, and the established treatment protocol involves controlling blood sugar levels with either daily injections of insulin or continuous oral hypoglycemic drugs [5]. Various factors contribute to the development of T1D, including genetic, environmental, and autoimmune factors. Accordingly, the mechanism of T1D is complex and remains to be fully elucidated.
Type 1 diabetes is associated with autoimmunity, which is characterized by the immune-mediated breakdown of tolerance to the islet antigens, resulting in uncontrolled T cell-mediated autoimmune destruction of pancreatic β cells [2]. The most important of these immune cells are macrophages and T cells, which both play an important role in T1D [1, 6, 7]. Recent studies have shown that interleukin 35 (IL-35) is involved in the pathogenesis of diabetes by regulating the polarization of macrophages, thus improving blood glucose.
Interleukin 35, a recently discovered member of the IL-12 family, is a heterodimeric cytokine composed of Epstein- Barr virus-induced gene 3 (EBI3) and IL-12p35 [8]. It is secreted primarily by regulatory T (Treg) cells (CD4+CD25+) [9] from the differentiation of conventional CD4+ T cells that have a pivotal role in immune homeostasis, regulatory B cells, dendritic cells, and a small amount of activity in endothelial and muscle cells, monocytes, and cancer cells. IL-35 converts Treg cells into induced regulatory T cells (iTr35) and mediates their suppression exclusively. Therefore, IL-35 has a crucial role in maximizing the inhibitory effect of Treg cells [10]. IL-35 exhibits only immune-suppressive properties and has been shown to convey protection in immune-related diseases. Collison et al. [11] first demonstrated, in an inflammatory bowel disease (IBD) model, that the inhibitory effect of Treg cells on effector T cells (Teff cells) is greatly reduced by the deletion of the two subunits, EBI3 and P35. Researchers also observed that CD4+CD25 iTr35 cells stimulated by exogenous IL-35 could have an immuno-suppressive effect in an IBD mouse model [9]. In Graves’ disease (GD), the serum level of IL-35 was reported to be reduced in a patient with GD compared to the controls, as determined by the ELISA method, which suggested that IL-35 may play an anti-inflammatory role in GD [12]. In recent years, IL-35 has also been found to have a protective effect in diabetes. By searching the keywords IL-35 and diabetes through the PubMed medical literature retrieval website, 32 papers describing the relationship between IL-35 and diabetes were found. In this review, we focus on how IL-35 impacts the development of diabetes in various ways, highlighting both the protective effect and the mechanism in both human and animal studies.
Protection against diabetes conferred by interleukin 35
The study of IL-35-induced immunomodulatory protection with regards to autoimmune and anti-inflammatory interactions may contribute to the development of new therapeutic approaches to inflammatory diseases. IL-35 therapy can ameliorate or prevent some autoimmune diseases, such as IBD, rheumatoid arthritis, colitis, psoriasis, and asthma. This is consistent with an analysis of the efficacy of pentosan polysulfate sodium (PPS) on a dextran sulfate sodium (DSS)-induced colitis model; PPS was reported to promote mucosal proliferation and repair by increasing the protein level and gene expression of IL-35 [13]. Furthermore, Xin et al. found that IL-35 can mitigate the progression of rheumatoid arthritis, most notably through maintaining the balance of Treg cells and Th17 cells [14]. Peng et al. reported that recombinant IL-35-BCG can regulate the Treg/Th17 cell imbalance and inflammatory response in asthmatic newborn mice induced by respiratory syncytial virus (RSV) infection through the c-Jun N-terminal kinase (JNK) signaling pathway, suggesting a new pathway to target in neonatal asthma treatment [15]. Recently, IL-35 has been tested in vitro and in vivo to explore its potential application in the treatment of T1D.
Several animal models of T1D, including a non-obese diabetes (NOD) mouse model, a multiple low dose streptozotocin (MLDSTZ)-induced T1D model, and a genetically engineered mouse model of T1D, and analyses of human peripheral blood obtained from patients with T1D and healthy controls are currently available. The mechanism of IL-35 in protecting and preventing T1D may be through inhibiting the proliferation and infiltration of immune cell in T1D models and patients. Bettini et al. [16] demonstrated that IL-35 protects NOD mice from autoimmune attack through the establishment of a NOD RIP-IL35 transgenic mouse model, with wild-type littermates as a control. They found that the number of CD4 and CD8 T cells infiltrating the islet cells in NOD RIP-IL35 mice decreased significantly, along with the frequency of islet antigen-specific T cells, which ultimately played a role in limiting the development of autoimmune diabetes. Further, the ectopic expression of IL-35 in β cells may protect against autoimmune diabetes, and the continued expression of IL-35 in β cells may be necessary to inhibit immune cell infiltration and prevent complications of diabetes. This transgenic technology of transplantation of islets or β cells in patients with diabetes protects inhibitors from autoimmune damage [16]. Mondanelli et al. observed that [17], around the eighth week, NOD mice in the dendritic cell group that received pulse IGRP peptide (a major diabetic autoantigen in NOD mice) had a higher incidence of diabetes than those receiving IGRP-pulsed dendritic cells (DC35). In contrast, the IGRP-pulsed DC35 cell group maintained normal blood sugar until the 10th week, after which the incidence of diabetes increased but did not exceed 25%. At the same time, the study showed that the ectopic expression of IL-35 by dendritic cells increased the number of Treg cells in NOD mice on the 21st day after treatment. Thus, DC cell therapy based on IL-35 Ig-producing and IGRP-presenting DCs may efficiently protect prediabetic NOD mice from developing hyperglycemia and overt diabetes. Subsequently, the same conclusion was obtained in 12-week-old NOD female mice injected with AAV8mIP-IL-35, which had the potential to inhibit β cell autoimmunity, hence suppressing the progression of overt diabetes; this provided evidence for the injection of exogenous IL-35 in a T1D mouse model as a novel anti-diabetes therapy [18]. These data were consistent with Singh et al., who found that transgenic IL-35 expression targeted against β-cells in NOD mice improved inflammation around the islets, preventing the occurrence of primary and secondary T1D, which inhibited the proliferation and infiltration of CD4+ T cells, CD8+ T cells, Foxp3 Treg cells, and regulatory B cells (Bregs) around the islets of NOD mice in the MLDSTZ-induced T1D model [19]. After systemic administration of recombinant IL-35, compared to treatment with phosphate buffer saline (PBS), the former remained normoglycemic with a lower insulitis status while the latter mice became hyperglycemic from day 10 and developed moderate insulitis. However, once IL-35 therapy was limited, diabetes developed. Furthermore, they drew the same conclusion from the NOD mouse model as that from the MLDSTZ T1D model, such that compared with age-matched wild-type CD-1 mice, the proportion of Foxp3 Treg cells was higher in pancreatic draining lymph nodes (PDLNs) of NOD female mice with prediabetes (13-15 weeks of age). Luo et al. [20] observed that IL-35 treatment prevented a decrease in Breg and IL-35+ Breg cells in STZ-induced diabetic mice, which effectively maintained an anti-inflammation phenotype in the T1D mouse model. These data showed that the IL-35 response in the MLDSTZ mouse is in line with the NOD model. In an analysis of human peripheral blood obtained from patients with T1D and healthy controls, insufficient IL-35 levels were reported, and IL-35 expression was significantly decreased in the serum of C-peptide negative patients with T1D in contrast with C-peptide positive patients with T1D [21]. Singh et al. noted that IL-35+ regulatory T cell frequency and plasma IL-35 concentration in patients with late autoimmune diabetes in adults (LADA) were lower than those in healthy controls [22]. Ouyang et al. reported that serum levels of IL-35 in patients with T1D were lower than in healthy controls [23]. These results indicate that IL-35, expressed in local islets, can regulate the immune response of T1D by controlling the immune response of CD4+ and CD8+ T cells. Moreover, these results provide a theoretical basis for the injection of exogenous IL-35 into patients with T1D as a novel anti-diabetes therapy. The protective role of IL-35 in T1D is shown in Table 1.
Table 1
Protective role of IL-35 in type 1 diabetes (T1D) in NOD mice, STZ mice, and T1D patients
Mechanisms of interleukin 35-dependent protection against diabetes
While the pathogenesis of T1D is unclear, we know that the immune system is involved [24]. The characteristic immune response of IL-35 that protects against diabetes includes the macrophages and the adaptive immune response, such as tumor necrosis factor α (TNF-α), Th1, Th17, Treg, iTr35, and Breg. Th1 cells are involved in the destruction of islet β cells, and polarization of Th1 immunity is a prerequisite for T1D development. According to one study, therapeutic agents targeting CD26 by suppressing T-cell proliferation and Th1 cytokine production regulate autoimmunity in T1D [25]. TNF-α participates in the development of impaired islets in the pathogenesis of T1D, which is one of the most important pro-inflammatory mediators. A raised level of TNF-α induces inflammation around islets in adipocytes and peripheral tissues and can accelerate the course of T1D by impairing insulin signaling through serine phosphorylation [26]. IL-35 is involved in many mechanisms to protect against diabetes, among which is macrophage polarization where Treg and Th1 are the most important.
Polarization of macrophages
Macrophages can be affected by various factors, which can alter the phenotype and thus function of macrophages; activated macrophages are usually divided into two categories, namely, M1-like macrophages and M2-like macrophages, which are closely related to pro-inflammatory and anti-inflammatory responses, respectively [27]. Compared with control animals [28], STZ-induced macrophages in T1D mice showed higher levels of inflammatory cytokines/chemokines, nitric oxide (NO) secretion, NLRP3 and iNOS protein levels, and enhanced glycolysis activity. Improving the inflammatory environment by modulating the activation state of macrophages is an effective method for the treatment of diseases [29]. TNF-α and IL-10 are stimulated after T1D islet transplantation and are expressed in the blood, causing macrophages to change phenotype and content to M2. This then improves immune tolerance, thus promoting islet neovascularization after transplantation; this eliminates apoptosis in pancreatic islet transplantation [30]. Some of the regulatory immune responses induced by IL-35 are dependent on macrophages. Zhang et al. [6] found that IL-35 plays an immune-suppressive role in psoriasis by decreasing the total number of macrophages and the ratio of M1/M2 macrophages. In 2019, Jiang et al. demonstrated that IL-35 is involved in the protection of diabetic neuropathic pain (DNP); they found that the mechanism underlying the therapeutic effect of IL-35 on DNP relates to the promotion of microglial polarization toward the M2 phenotype by inhibiting JNK signaling and Janus kinase-2/Signal transducer and activator transcription 6 (JAK2/STAT6) signaling [31]. All studies emphasized that modulating the macrophage phenotype might be useful to ameliorate immune-related diseases including diabetes. However, the molecular pathway of IL-35 in the protection of T1D through regulation of the macrophage phenotype remains to be explored.
Treg cells
Treg cells are essential for immune homeostasis. Tang et al. [32] reported that the mechanism by which the parasite ameliorates or prevents the development of T1D is particularly related to the activity of Treg cells. Treg cells are characterized by high expression of the transcription factor Foxp3, which releases inhibitory cytokines such as IL-10, TGF-β, and IL-35, as major regulators of Treg cell phenotype and function [33, 34]. Treg cells suppress autoimmunity, which is achieved to a large extent by blocking the T cell-mediated cycle and blocking Th1 and Th17 cell differentiation [18, 29]. Moreover, IL-35 promoted CD4+ T cell differentiation into iTr35 cells, which still possessed the ability to secrete IL-35 [10]. Over the past decade, a growing number of studies have indicated that IL-35 plays a crucial role in regulating Treg cells in immune-related disorders, including autoimmune diseases, infectious diseases, and cancer. In an asthma mouse model [16], IL-35 improved asthma symptoms by promoting the proliferation of Treg cells and inhibiting the differentiation of Th17 cells. In diabetic rats with impaired renal function, increased levels of IL-17A and decreased levels of IL-35 and IL-10 were observed; exogenous resveratrol therapy increased the expression of IL-35 secreted by Treg cells and improved renal function [35]. Moreover, Cao et al. [36] reported that IL-35 is vital for regulating Treg/Breg cell responses during the development and progression of GDM. In a model of islet transplantation [37], Treg cells inhibited Th17 proliferation in islet cells, which was enhanced by IL-35; IL-35 induced an increase in Treg cells, which may further contribute to the protective effect in transplanted islets. While IL-35 can promote differentiation of conventional CD4+ T cells into iTr35 cells [11, 38], IL-35 played a central role in inhibiting the proliferation and expansion of CD4+ and CD8+ T cells by partly regulating Foxp3+ and Treg cells, which are essential in the onset of diabetes. These results suggest that IL-35 may protect against the development of T1D through control of inflammation in circulatory and local metabolic tissues by Treg cells. Moreover, Treg cells may participate in the protective effect of IL-35 on diabetes, as shown in Figure 1.
Fig. 1
Possible mechanism of IL-35 protection against type 1 diabetes
IL-35 – interleukin 35, M1 – classically activated macrophage, M2 – alternatively activated macrophage, Foxp3 – Forkhead box protein P3, Treg – regulatory T cells, Bregs – regulatory B cells, Th1 – T helper 1, STAT6 – signal transducer and activator transcription, JAK2 – Janus kinase-2, IR – insulin resistance, T1D – type 1 diabetes, DNP – diabetic neuropathic pain
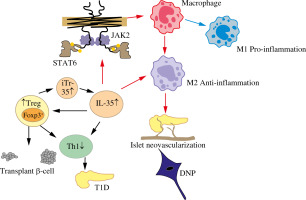
Th1
There is growing evidence to suggest that the Th1 immune response and related cytokines such as IFN-γ play an important role in the pathogenesis of T1D [39]. Kathamuthu et al. reported that when latent tuberculosis antigen stimulated diabetes mellitus (DM) and prediabetes (PDM), the levels of Th1-associated cytokines IFN-γ and TNF-α in cultured serum increased significantly in T cells [40]. Importantly, Treg cells can convert Th1 into Th2, secreting the cytokines IL-35, IL-10, and TGF-β to generate immune tolerance, ultimately treating and preventing the occurrence of T1D [32]. Chen et al. pointed out that the injection of exogenous IL-35 can stimulate an increase in Breg cells and can inhibit the proportion of Th1 [41]. In addition, Jiang et al. showed that IL-35 can also inhibit CD8+ T cell activity by inhibiting the expression of Th1 cytokines, hence serving as protection against T1D [42]. All of these contribute to preventing and treating T1D.
Conclusions
In this review, we have summarized the latest evidence on the involvement of IL-35 in protection against T1D and the mechanism behind it. IL-35, as an anti-inflammatory cytokine, improves T1D by regulating the polarization of macrophages and the proportion of T cell-related cytokines. This opens a new avenue to immunotherapeutic strategies for T1D. Despite compelling evidence from animal models supporting the potential role of IL-35 in modulating the course of T1D, the clinical translation of these findings is slow, and many obstacles persist. The ultimate goal is to identify a balanced, non-invasive, and effective means of decreasing the incidence of diabetes without affecting the fitness of an individual.


