Introduction
Secukinumab (SEC) is a fully human monoclonal antibody that specifically neutralizes interleukin-17A and has been shown to play an important role in the aetiology of psoriasis [1]. Clinical trials and real-world research have previously confirmed the long-term efficacy and safety in the treatment of psoriasis, including difficult-to-treat areas such as the nails, scalp, palms, soles, and joints [2–4]. It was approved in Turkey in March 2018 for the treatment of moderate to severe chronic plaque psoriasis.
Drug survival, also known as “drug retention” or “drug persistence”, refers to the length of time a patient continues to take a drug once it has been determined to be an effective form of therapy for that patient. It serves as an indirect measure of therapeutic success as it depends on the effectiveness of the treatment, the occurrence of undesirable effects and the tolerability of the drugs [5]. Additionally, the low survival rate of a drug is a major factor in the rising cost of treatment as induction protocols are required for each biological switch. Although SEC has been shown to be highly effective and safe in the treatment of moderate to severe plaque psoriasis (PsO), further real-world experience (RWE) is needed as there are conflicting results on SEC survival [5–17]. In addition, the results of previous studies show that there is a high degree of diversity in the factors that influence SEC survival.
Aim
In this retrospective study conducted in two tertiary outpatient dermatology clinics our aim was to analyse the survival rate of SEC and predictive factors of SEC survival for the treatment of psoriasis, together with the drug safety and efficacy.
Material and methods
This study is an extension of a retrospective cohort study that we conducted before [18]. The key difference between the current analysis and our earlier publication, aside from the longer time of follow-up with SEC, was that data from more patients receiving SEC were included, and this real-world study concentrated on SEC survival and its predictive factors. This study includes updated clinical data for SEC as well as a longer follow-up (our last study was concluded in April 2020).
Patients
This study included patients with chronic psoriasis with or without psoriatic arthritis who received SEC treatment in the outpatient clinics of Buca Seyfi Demirsoy Research and Training Hospital, Dermatology Clinic, İzmir, Turkey, and Sanko University, Department of Dermatology, Gaziantep, Turkey and who had to have at least one follow-up visit between May 2018 and April 2022. According to reimbursement policies in Turkey, patients with moderate to severe psoriasis with or without arthritis, could be treated with biologics if they had not responded to or had contraindications to at least one conventional treatment, such as systemic therapy or phototherapy. The research was conducted in accordance with the Declaration of Helsinki.
Treatment and outcomes
SEC was delivered (300 mg, s.c.) during weeks 0, 1, 2, 3, and 4 as a loading dose and subsequently once every 4 weeks. Concomitant treatment with methotrexate (MTX) 10–15 mg/week and acitretin 25–35 mg/day was commenced in patients who did not respond to therapy in the 16th or 28th week of treatment, or the dose of SEC was raised (300 mg every 2 weeks).
Patient demographic and clinical data (age, sex, height, weight, body mass index (BMI), age at onset and family history of psoriasis, disease duration, comorbidities, scalp, nail and genital involvement, previous systemic and biologic therapies), baseline, week 16, week 28, and week 52 psoriasis area and severity index (PASI) scores, and adverse effects experienced during therapy were recorded from data sheets. PASI was used to assess the severity of PsO at weeks 16, 28 and 52.
Rates of decrease in PASI scores were used to evaluate treatment effectiveness; a 75%, 90%, and 100% reduction in baseline PASI scores were labelled as PASI 75, 90, and 100 and reported at weeks 16, 28, and 52, respectively. Primary failure in our study was defined as failing to reach PASI 75 by at least week 16, and secondary failure was defined as first reaching PASI 75 but then permanently losing it. Both primary and secondary failure were categorized as lack of efficacy.
The occurrence of adverse events (AEs) over the study period was used to determine safety and tolerance.
Drug survival was assessed in months, which was defined as the duration between initiation and discontinuation of treatment. In this context, “discontinuation date” was defined as the day treatment was stopped or a different drug was given instead. If a patient stopped attending the hospital before the end of the study, the date of his or her final visit was designated as the date of discontinuation.
Statistical analysis
Descriptive statistics were used to calculate frequencies and percentages of categorical variables and quantitative variables were described as mean and standard deviations (SDs). Kaplan-Meier method was used to estimate survival probabilities. Univariate and multivariate hazard regression was performed to determinate possible risk factors for SEC survival. The following were considered as possible predictors: sex, age, obesity (body mass index (BMI) > 25 kg/m2), arthritis, previous use of biologics and comorbidity status, concomitant treatments, family history of psoriasis, C-reactivity protein (CRP) positivity, baseline PASI, QuantiFERON positivity, side effects. Variables which are significant according to univariate analysis and potential risk factors were included in the model. Variance inflation factors (VIF) were calculated to avoid any multicollinearity problem. All analyses were performed by using SPSS for Windows version 24. A p-value < 0.05 was considered as statistically significant.
Results
A total of 268 patients (59.3% male) were included in this study. The data collected from each patient at baseline are summarized in Table 1. The mean (± SD) age of the patients was 45.2 ±13.1 (range: 35–55 years) years and their mean disease duration was 14.53 ±11.11 years. Mean baseline BMI of the patients was 27.63 ±4.54 kg/m2. The associated comorbidity rate among our cohort had been 31.3% (n = 84). At least one comorbidity was present in 51 of the patients; 22 had two; and 11 (4.1%) were diagnosed with three or more comorbidities. The baseline mean (SD) PASI at the initiation of SEC treatment was 18.44 ±8.86, and all of the patients had previously been treated with different conventional systemic therapies. PsA was detected in 32 (11.9%) of the patients. A total of 41 (15.3%) patients had previous exposure to biologics. Patients who failed to reach PASI 50 after 16 or 28 weeks of treatment were given concomitant medications (MTX 10–15 mg/week (n = 42,15.7%) and acitretin 25-35 mg/day (n = 9, 3.4%)), or the dosage of SEC was increased to 300 mg every 2 weeks (n = 26, 9.6%).
Table 1
Demographic features and clinical characteristics
Treatment efficacy
PASI 75/90/100 was observed in 89.5 % (221/247), 78% (198/247), and 16.2% (40/247) of patients at week 16, 94.8% (254/268), 85.4% (229/268), and 12.3% (33/268) of patients at week 28, and was well maintained in patients who reached week 52, with 96.3% (206/214), 90.7% (194/214), and 15.4% (33/214), respectively. 16 week, 28 week and 52 week mean (± SD) PASI scores were 1.97 ±3.33, 1.51 ±2.33 and 1.31 ±2.05, respectively.
Drug survival
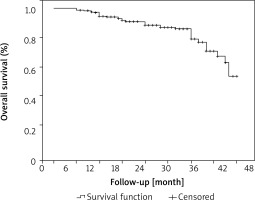
The observed cumulative drug survival probability rates for SEC were 94.4% at 12 months, 88.4% at 24 months, 78.6% after 3 years, 52.7% after 4 years (Figure 1). The mean overall survival time was 41.60 ±0.86 months for all reasons of discontinuation. During the study period, 41 (15.3%) discontinuations were identified, and the reasons for discontinuation are described in Table 2. Lack of efficacy was found in 17 patients (6.3%; primary and secondary failure was found in 5 and 12 patients, respectively). Primary failure was caused by treatment-resistant psoriasis skin lesions in three individuals, arthritis-related symptoms in one, and palmoplantar psoriasis lesions in one. Five patients were lost to follow-up. In 14 patients, treatment was discontinued due to their own decision. Notably, 4 patients discontinued treatment due to complete remission of psoriasis-related symptoms. One patient stopped SEC due to frequent recurrent upper respiratory tract infections and 2 patients discontinued SEC due to their decision to become pregnant. One cause for discontinuance was mortality owing to aortic aneurysm in the study cohort.
Table 2
Reasons for SEC discontinuation (n = 41, 15.3%)
Figure 1
Overall cumulative drug survival of SEC in psoriasis patients represented by Kaplan-Meier plot. Data show all-cause discontinuation
The multivariate analysis of factors related to SEC survival (Table 3) showed that concomitant systemic treatment (HR = 2.42, 95% CI: 1.12–5.2; p = 0.024) and family history of psoriasis (HR = 3.1, 95% CI: 1.24–7.79; p = 0.016) was associated with a reduced SEC survival. Univariate analysis showed SEC updosing was associated with a reduced SEC survival (HR = 2.84, 95% CI: 1.47–5.49; p = 0.002). The other variables analysed (gender, age, baseline PASI, CRP positivity, QuantiFERON positivity, previous biologic exposure, BMI, comorbidity, arthritis, adverse events) were not significantly associated with drug survival.
Table 3
Analysis of several factors that can potentially relate to drug survival (Cox regression model)
Safety
Adverse events (AE) were reported in 40 (14.9%) patients in this study cohort (Table 4). The most often reported AEs in the study population were infection-related (n = 21, 7.8%). The most prevalent infection-related AEs were oral (n = 7) and vaginal (n = 4) candida infections, followed by folliculitis (n = 3), pneumonia (n = 3), recurrent upper respiratory tract infections (n = 1), urinary tract infection (n = 1), soft tissue infection (n = 1), and acute vestibular neuritis (n = 1). Interestingly, among non-infectious reasons, pruritus (n = 6) and dermatitis-like lesions (n = 3) were the most frequent causes of AEs. Both diarrhoea (n = 1) and injection site reactions (n = 1) were uncommon. Three patients experienced major cardiovascular events (MACE), including 2 cases of acute myocardial infarction and one death owing to an aortic aneurysm which were not considered by the investigators to be related to the study drug. Recurrent upper respiratory tract infections (URTI) in 1 patient were the cause of treatment discontinuation owing to AEs.
Table 4
Adverse events during SEC treatment
Discussion
This retrospective study cohort of 268 psoriasis patients provides data on the real-life efficacy, long-term survival, adverse events related to SEC treatment, and predictive factors for SEC survival.
PASI 75/90/100 responses achieved at week 16 (89.5%, 78%, and 16.2%, respectively) were well maintained at week 52 (96.3%, 90.7%, and 15.4%, respectively) in this study cohort. At week 52, the rates of PASI 75 and PASI 90 were higher compared to those found in our previous study (86.1% and 64.6% respectively), as well as in earlier RWE findings and clinical trials [2, 16–20]. Age, gender, BMI, and disease duration were all comparable when the demographics of the participants of our prior study were compared with the current study. However, this cohort’s mean baseline PASI (18.44) was lower than the prior cohort’s mean baseline PASI (20.8). In addition, there were more comorbidities in our previous study (43.8% vs. 31.3%). Additionally, compared to the last cohort, our current group had a higher percentage of naive patients (84.7% vs. 79.7%). Our current study cohort contained more naive psoriasis patients with less concomitant diseases and lower baseline PASI values, which may have contributed to the higher PASI 75, 90 and 100 rates. On the other hand, PASI 100 rates in this current study similar to our previous study were lower when compared to earlier clinical studies and RWE [2, 18–21]. Disparities in efficacy results may be attributed to patient variables (mean age, race, sex, baseline comorbidities and BMI). Additionally, not all patients had their PASI values assessed on a regular basis since they were unable to attend their scheduled follow-up appointments because of concerns regarding COVID-19. When we compared the demographics of clinical trial patients with our cohort, we observed that the present study had more female patients (40.7% vs. 23–36% in trials) and that patients were of a similar age (mean 45.2 years, vs. 43.9–46.6 in trials) [19–21]. The SEC clinical trials’ mean baseline PASI, which ranged from 18.9 to 23.9, was comparable to our cohort’s mean baseline PASI of 18.44 [19–21]. Similar to our prior study cohort, this cohort’s mean PASI score at baseline (18.44) was greater than that of previous real-life studies [2, 18]. As we stated in our earlier study, reimbursement for biologic drugs in Turkey is contingent on the use of at least one conventional systemic medication. We believe that the fact that all patients had previously used conventional medicines contributed to their substantially reduced PASI 100 rates. Our results, however, are in line with other research showing that SEC has a significant effect on reducing disease severity quickly and exhibits sustainable efficacy over time [2–4, 19–21].
The observed cumulative drug survival probability rates for SEC were 94.4% at 12 months in this study. The drug survival curve in our analysis did not show a sharp decline in SEC efficacy. Instead, drug survival gradually decreased after 12 months of therapy due to discontinuations that occurred during the follow-up period. In comparison to previously reported RWE regarding cumulative drug survival rates, which vary from 72% to 93% [5–17], SEC’s 12-month overall cumulative drug survival was higher in this trial. Compared to previous drug survival studies, the limited percentage of biologic experienced patients in our cohort (15.3%) may partially explain the improved survival rates shown in our research. The majority of recent studies have shown that biologic-experienced patients have a shorter SEC survival rate than non-experienced patients, supporting our theory [5–8, 13–17, 22, 23]. Furthermore, the close follow-up that patients received, dose escalation, and the use of concomitant medication, which helped to maintain or recover a good response in terms of PASI 75, may have contributed to the high survival rates with SEC therapy at 12 months. The longer-than-3-year survival of SEC is yet inadequately investigated. In our study, the cumulative drug survival probability rates for SEC were 88.4% after 2 years, 78.6% after 3 years, and 52.4% after 4 years. Sotiriou et al. [17] reported that the overall medication survival rate for SEC was 83.1% after 2 years, 75.4% after 3 years, and 72.7% after 4 years. Similar to our analysis of drug survival, their study indicated that drug survival declined progressively over time rather than abruptly.
In this study, concomitant systemic therapy, updosing and a family history of psoriasis were associated with decreased SEC survival according to the analysis of covariates related to SEC survival. Surprisingly, high BMI and prior biologic exposure, which have been linked to drug survival in several prior RWE studies, were not related with SEC survival in our investigation [5–8, 13–17, 22, 23]. However, we believe that this outcome was attained because the majority of the research participants who received concomitant drugs and higher doses were biologically experienced and difficult-to-treat patients. In agreement with our findings, Torres et al. [6] found that patients treated with concomitant systemic medications had a lower drug survival rate at 12 months (79.5% vs. 94.2%, respectively) and 18 months (53.7% vs. 81.2%, respectively) than those treated with SEC alone. Female sex has been linked to SEC survival by some previous research [8, 10]; however, our study’s analysis of variables associated to SEC survival found no such association similar to Ortolan et al. [13]. The necessity for further RWE studies is highlighted by the literature’s great degree of variety in the factors that affect SEC survival.
Discontinuations were primarily due to primary or secondary failure (n = 12, 6.4%), rather than AEs (n = 2, 0.7%), which is consistent with prior research [2, 3]. AE were documented in 40 (14.9%) patients in this study cohort, which is comparable to clinical trial rates [3]. In this study, the most prevalent adverse events were infection-related, with candida infections (n = 11, 4.1%) being the most common infectious cause. Although somewhat higher than the published clinical trial rates (1.7%) [24, 25], none of the candidiasis instances were serious, and none led to cessation of the treatment. Interestingly, pruritus and dermatitis-like lesions were the second most common adverse events and observed in 6 patients which has also been previously reported by Wang et al. [11]. Atopic like dermatitis secondary to IL-17 inhibitor treatment has been linked with the role of IL-17 in eczema pathogenesis [25].
Although the main limitation of our study is its retrospective nature, it provides valuable data on the long-term drug survival of SEC improving the RWE data.
Conclusions
Our findings in this retrospective, long-term study validated prior findings on the quick action of SEC and its stable effectiveness over time. We found that overall cumulative drug survival for SEC was 94.4% at 12 months, 88.4% at 24 months, 78.6% after 3 years, 52.7% after 4 years. Additionally, concomitant treatments, dose escalation and family history of psoriasis were associated with a higher risk for SEC withdrawal. Drug ineffectiveness was the main reason for drug discontinuation. Our results improve previous RWE findings regarding the long-term drug survival of SEC. In patients with psoriasis who are particularly challenging to treat and who require dose escalation and concomitant medications, close monitoring may improve drug survival.








