Introduction
Immune response (IR) refers to the process whereby immune cells recognize, activate, proliferate, differentiate antigen molecules, produce immune substances and generate specific immune effects under stimulation of antigens. The immune response can be activated or inhibited in the body, which are balanced by substances ensuring immune responses to pathogens in the immune system, such as leukocyte immunoglobulin-like receptors (LILRs). Leukocyte immunoglobulin-like receptor B4 (LILRB4) belongs to the LILR family, which is widely distributed on immune cell membranes. LILRB4 is a kind of inhibitory receptor that plays a key role in immune checkpoint pathways and participates in regulating multiple immune diseases [1].
The structure of LILRB4
LILRB4 is a member of the leukocyte immunoglobulin-like receptor (LIR) family, which is found in a gene cluster in chromosomal region 19q13.4 [2, 3]. According to the role of intracellular motifs, immunoglobulin-like transcripts (ILTs) were divided into the activated receptor LILRA and the inhibitory receptor LILRB. The activating receptor LILRA includes six receptors, LILRA1-LILRA6, while the inhibitory receptor includes LILRB1-LILRB5 [4, 5]. Inhibitory LILR members have a long cytoplasmic tail containing different sets of immunoreceptor tyrosine-based inhibitory motifs that recruit phosphatases and thus contribute to downstream inhibitory signaling pathways [6, 7]. Activating members on the other hand have truncated cytoplasmic regions and are thought to associate with activating adaptor proteins via a positively charged amino acid in the transmembrane domain (e.g. LILRA2 and LILRA4 both associate with the γ chain of FcεRI) [8, 9]. LILRBs contain two or four extracellular immunoglobulin domains, a transmembrane domain, and two to four cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIMs) [10]. Interestingly, LILRB4 is somewhat unusual as whereas most family members contain four immunoglobulin-like domains in their extracellular region (designated D1, D2, D3, and D4) LILRB4 is one of two members that only possess two immunoglobulin-like domains (the other one is the closely related activating receptor LILRA5) [11].
The function of LILRB4
The immune system is regulated by LILRB4, which is produced on immune cells and may bind to MHC class I molecules on antigen-presenting cells as well as other ligands such as integrins αVβ3, apolipoprotein E, and fibronectin [12-15]. Furthermore, it can also function in antigen capture and presentation. Previous studies have indicated that ILT3-Fc acts through BCL6 and is a potent immunosuppressive agent for reversing the onset of allo- or possibly autoimmune attacks against pancreatic islets [16]. It is thought to control inflammatory responses and cytotoxicity, which is helpful to focus the immune response and limit autoreactivity. Chang et al. found that ILT3-Fc inhibits T cell activation and induces the generation of Ts targeting multiple inflammatory miRNA pathways [17]. Simultaneously, LILRB4 can regulate marginal zone B cells and antibody production [18]. In cardiomyocytes, LILRB4 can regulate apoptosis and mediate myocardial hypertrophy [19].
Regulators of LILRB4 expression
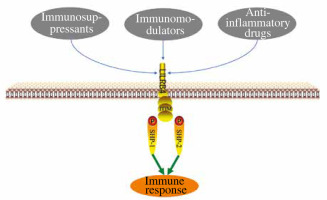
LILRB4 has been proven to play an important role in the immune system. There are three main substances that can regulate LILRB4 expression: immunosuppressants, immunomodulators and anti-inflammatory drugs (Fig. 1). Penna et al. detected a significant increase in the expression of ILT3 after treatment with 1,25(OH)2D3, and proved that 1,25(OH)2D3 can induce the production of regulatory T cells through ILT3-dependent and non-ILT3-dependent pathways, while ILT3-dependent pathways are necessary for the induction of CD4+Foxp3+ regulatory T cells [20] (Table 1). Rochat et al. found that vitamin D supplementation during pregnancy was associated with increased ILT3 gene expression in a prospective trial [21]. However, Waschbisch et al. found that interferon-β alone or in combination with vitamin D could induce upregulation of ILT3 in vitro [22]. Interferon treatment led to a significant increase in monocyte ILT3 in vitro, and dihydroxyvitamin D3 also enhanced ILT3 expression. Švajger et al. found similar synergies between interferon (IFN)-γ and 1,25(OH)2D3 [23]. These results suggest that vitamin D receptor (VDR) agonists, alone or in combination with other immunomodulators, could up-regulate LILRB4 and contribute to DC tolerance. Some members of the interferon family also upregulate LILRB4 expression. Inui et al. found interleukin (IL)-2 to be an effective inducer of B4+CD38+ cells, and IFN-α is the main inducer of B4+CD38+ cells. The IFN-α pathway is involved in the pathogenesis of systemic lupus erythematosus (SLE) and IFN-α induces LILRB4 expression through plasmacytoid dendritic cells (PDC) and monocytoid dendritic cells (MDCs) [24]. After interferon treatment for 48 h, ILT3 expression was increased on freshly isolated purified monocytes and on immature myeloid dendritic cells derived from blood monocytes of multiple sclerosis (MS) patients and controls [25]. When ILT3 was blocked by anti- ILT3 antibodies, mitogen-driven proliferation of CD4+ T cells was increased several times in IFN-β treated relapsing-remitting MS (RRMS) patients and healthy controls, which may be involved in the inhibition of T cell activation in vivo. Immunosuppressants can also up-regulate LILRB4 expression in kidney transplant patients with chronic allogeneic nephropathy [26]. The infiltration of renal tubule interstitium and the level of ILT3 in endothelial cells were significantly increased, leading to reduction of CD40 in BDCA2+ cells by rapamycin and increase of Treg number, as well as expansion of the CD8+CD28+ T cell population. It was found that the RNA and protein levels of ILT3 and ILT4 in NKL cells increased 12, 24 and 36 h after cyclosporin A (CsA) treatment, and the killing activity of NKL cells towards tumor cells decreased significantly after CsA injection (15 mg/l) for 36 h. Thus, CsA up-regulated the expression of ILT3 and ILT4 in NKL cells, and affected its killing effect on tumor cells with different human leukocyte antigen G (HLA-G) expression and NKL cell proliferation [27]. Svajger et al. found that resveratrol could induce ILT3 expression, resulting in differentiation of dendritic cells (DCs) derived from human peripheral blood mononuclear cells (PBMCs) [28]. HLA-G exerts its inhibitory functions via interaction with ILT2, ILT4, and KIR2DL4, which are differentially expressed by NK, T, and antigen-presenting cells. ILT2, ILT3, ILT4, and KIR2DL4 expression is up-regulated by HLA-G in antigen-presenting cells, NK cells, and T cells, which may not need antigenic costimulation, possibly before the immune response [29]. In addition to immunosuppressants, some anti-inflammatory drugs can also regulate LILRB4 expression. Buckland et al. found that aspirin can induce the production of tolerant DCs, which may be related to the significant expression of ILT-3. Aspirin inhibits nuclear factor κB (NF-κB) translocation to the nucleus and induces antigen-specific Foxp3 positive regulatory T cells. Another nonsteroidal anti-inflammatory drug, niflumic acid (NFA), also upregulates ILT3 expression and participates in the development of immune tolerance in human monocyte derived DCs [30]. Brenk et al. [31] isocytes from peripheral blood of healthy subjects and cultured them under normal (30 µM) and low (5 µM) Trp (DCs+Trp and DCslow-TRP) conditions. The expression of ILT3 and ILT4 increased significantly in the DCslow-TRP group. The addition of anti-ILT3 mab partially restored the stimulation activity of DCslow-Trp on T cells, but had no effect on the stimulation of DC+Trp on T cells. These findings suggest that DCs with low tryptophan can induce high expression of inhibitory receptors ILT3 and ILT4, and increase inhibitory CD4, CD25 and Foxp3T cells in an ILT3-dependent manner, and weaken the stimulation ability of CD4+ T cells, thus leading to the immune tolerance of DCs. Interleukin 10 could inhibit endothelial dependent T cell costimulation by up-regulating ILT3/4 in human vascular endothelial cells [32]. In summary, LILRB4 has a variety of ligands, the discovery of which will provide a new therapeutic target for LILRB4 related diseases.
Table 1
Regulators of LILRB4 expression
| Regulators | Cell distribution | Effect |
|---|---|---|
| 1,25(OH)2D3 [20] | Regulatory T cells | Upregulation |
| Interferon β [25] | Monocyte | Upregulation |
| Interleukin 2 [24] | B4+CD38+ cells | Upregulation |
| Interferon α [24] | B4+CD38+ cells | Upregulation |
| Rapamycin [26] | CD8+CD28+ T cell | Upregulation |
| Cyclosporin A [27] | NKL cell | Upregulation |
| Resveratrol [28] | Dendritic cells | Upregulation |
| Human leukocyte antigen G [29] | NK cells, T cells | Upregulation |
| Aspirin [30] | Dendritic cells | Upregulation |
| Tryptophan [31] | Dendritic cells | Downregulation |
| Interleukin 10 [32] | Human vascular endothelial cell | Upregulation |
LILRB4/SHP-1 or SHP-2
LILRB4 has three ITIMs. The ITIMs of LILRB4 can recruit src homology 2 (SH2) domain-containing phosphatase 1 (SHP-1) and SHP-2 from the cytosol, leading to the activation of SHPs and the subsequent inhibition of various downstream signaling pathways [33]. Li et al. reported that ILT3 promotes tumor motility and angiogenesis via recruitment of SHP2/SHIP1 and subsequent activation of the ERK1/2 signaling pathway [34]. By interaction with its ligand ApoE, ILT3 induced tumor cell migration and invasion as well as tumor angiogenesis via activation of the SHP-2/SHIP1-ERK1/2 signaling pathway, which subsequently promoted EMT and the expression of VEGF-A, leading to non-small cell lung cancer (NSCLC) metastasis. Truong et al. demonstrated that the chicken LILRB4-5 genes activate the JAK/STAT signaling pathway, which plays a key role in multiple cytokine-induced responses [35]. In addition, LILRB4 can recruit SHP1 to inhibit TRAF6 ubiquitination and subsequently inactivate NF-κB and MAPK cascades [36]. Blocking TRAF6 ubiquitination to inactivate downstream MAPK and NF-κB signaling largely explained the inhibitory effect of LILRB4 on nonalcoholic fatty liver disease (NAFLD) progression. NF-κB plays a key role in the cellular inflammatory response and immune response. LILRB4 regulated NF-κB by recruiting SHP-1 or SHP-2 in turn affects atherosclerosis and myocardial hypertrophy [37, 38]. In leukemia, inhibition of NF-κB signaling reversed T cell suppression and reduced AML cell infiltration in a LILRB4-dependent manner. Urokinase-type plasminogen activator receptor (UPAR), an NF-κB target, is known to promote cancer invasion, metastasis, survival and angiogenesis [39]. The urokinase-type plasminogen activator (uPA) system is a biomarker and therapeutic target in human malignancies. It was found that ARG1 is a key downstream effector of LILRB4/NF-κB/uPAR signaling. LILRB4/SHP-2/NF-κB/uPAR-ARG1 could suppress immune activity and supports leukemia migration [40].
Relationship with disease
As an immune checkpoint, LILRB4 is of great significance in the treatment of autoimmune diseases [41]. Jensen et al. revealed that ILT3 loss of function polymorphism was associated with the increase of inflammatory cytokine level in SLE [42]. In addition to SLE, LILRB4 is also associated with a variety of immune diseases, such as Kawasaki disease [43] and allergic diseases [18]. LILRB4 is also closely related to diseases of other systems. By inhibiting NF-κB signaling pathway, LILRB4 can attenuate cardiac hypertrophy and atherosclerosis [44-46]. The maturation and migration of pulmonary DCs to lymph nodes in response to Ag and the innate immune stimulus is associated with upregulation of LILRB4. LILRB4 could attenuate the ability of DCs to elicit pathologic Th2 pulmonary inflammation [47]. Myeloid-derived suppressor cells (MDSC) accumulate in the tumor microenvironment (TME); they are engaged in tumor-associated immunosuppression, and govern tumor growth and metastasis. It was shown that LILRB4 can facilitate tumor invasion and migration by controlling MDSC and preventing the release of miRNAs from the miR-1 family, hence facilitating MDSC-mediated tumor metastasis [48]. ILT3 expressed on M-MDSCs can induce immunosuppression in cancer, and antagonism of ILT3 may be useful to reverse the immunosuppressive function of M-MDSCs and enhance the efficacy of immune checkpoint inhibitors [49]. Sharma et al. also revealed that LILRB4 strongly suppresses tumor immunity in TME [50]. The above evidence indicates that LILRB4 may be an effective target for cancer treatment. LILRB4 may also be a potential drug for the treatment of autoimmunity and graft rejection [51]. For example, LILRB4 is an effective immunomodulator for inhibiting allograft rejection in islet allograft [52]. Furthermore, it is presumed that LILRB4 also participates in regulating central nervous system immune surveillance. Recombinant human ILT3.Fc protein can bind to murine immune cells and further inhibit the release of proinflammatory cytokines, which suggests the feasibility of ILT3 in treatment of multiple sclerosis [53].
Conclusion and perspectives
LILRB4 was first found on the immune cell membrane and gradually came into people’s vision. Current studies prove that LILRB4 not only acts on tumors and immune diseases, but also diseases of multiple systems, which indicates that LILRB4 has great potential in disease treatment. The understanding of the LILRB4 extracellular ligand and downstream signal pathway can deepen the understanding of LILRB4 in physiology and pathology, and help LILRB4 become a new biomarker of disease. However, it is still necessary to study LILRB4 further, especially at the cellular and molecular levels.