Case report
Here, we describe a case of a 52-year-old man with long-standing ankylosing spondylitis (AS) with bilateral ankylosis of the sacroiliac joints, grade IV according to modified New York criteria (Fig. 1) with a history of peptic ulcer disease and no other comorbidities who developed seropositive rheumatoid arthritis (RA). The diagnosis of AS was made in 2006 and was based on a combination of inflammatory back pain, X-ray of the sacroiliac joints (bilateral grade III sacroiliitis at the time of diagnosis) and the presence of HLA-B27. He did not present peripheral arthritis, enthesitis, uveitis or psoriasis. At the time of diagnosis, inflammatory indicators were increased: C-reactive protein (CRP) 9.8 mg/l (normal value [NV] < 5 mg/l), erythrocyte sedimentation rate (ESR) 48 mm/h (NV < 12 mm/h]) but rheumatoid factor (RF) and anti-citrullinated peptides antibodies (ACPA) were absent. Baseline disease activity measured with Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) was high: 8.6. Initially, the patient was treated with non-steroidal anti-inflammatory drugs (NSAIDs) for 6 years. Since NSAIDs ceased to control the disease activity in 2012, etanercept was introduced. He had undergone left (2013) and right (2014) hip arthroplasty due to severe secondary coxarthrosis in the course of AS. Because of worsening of spinal pain and stiffness, significant deterioration in mobility, and persistent systemic inflammation (ESR 52 mm/h, CRP 102 mg/l) etanercept was replaced with secukinumab in January 2020. In February 2020, after two doses of secukinumab, the patient was admitted to the tertiary rheumatology center with acute symmetric polyarthritis of new-onset involving shoulders, elbows, wrists, small joints of the hands, feet, and knees with pain scored 10/10 on the Visual Analogue Scale (VAS). Inflammatory indicators were increased: CRP 35 mg/l, ESR 73 mm/h. X-rays of the hands and feet did not reveal abnormalities. Ultrasound of the joints revealed (according to the OMERACT recommendations): GS = 1, PD = 1 bilaterally in the wrist joints, GS = 1 + effusion, PD = 0 (left hand III PIP); tenosynovitis in the fourth extensor compartment of the right hand (GS = 1 + effusion, PD = 0). Systemic steroid therapy was started and NSAIDs were maintained, resulting in a transient clinical improvement. At 2 months of follow-up polyarthritis with symmetric involvement of the wrists was still present. Autoantibodies were detected: ACPA 500 IU/ml (NV < 8), RF 379 IU/ml (NV < 15). Therefore, the patient was diagnosed with RA according to the ACR/EULAR 2010 criteria [1]. Treatment with the JAK inhibitor tofacitinib was initiated in July 2021. Despite treatment, symmetric polyarthritis of the hands persisted. Follow-up X-rays of the hands showed juxta-articular osteoporosis and inflammatory cysts in the joints that were most affected by ultrasound (Fig. 2). In December 2021 tofacitinib was switched to anti-interleukin (IL)-6 tocilizumab, which caused substantial clinical improvement and allowed remission of RA along with sustained remission of AS. Treatment modifications and disease activity are presented in Table 1. Comparison of RA and AS diagnosis and treatment is presented in Table 2. After 18 months of tocilizumab treatment, both RA and AS remain in remission (DAS-28 1.2, BASDAI 2.0, ESR 1 mm/h, CRP 1 mg/l).
Fig. 1
X-ray of sacroiliac joints showing bilateral ankyloses typical for final stage of ankylosing spondylitis and two hip prostheses. The prostheses were implanted because of severe hip joint destruction caused by ankylosing spondylitis
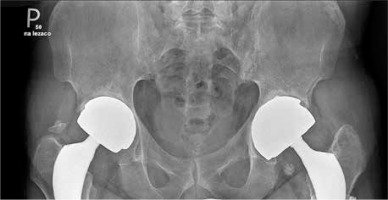
Fig. 2
X-ray of hands showing juxta-articular osteoporosis and inflammatory cysts. No erosions and joint space narrowing. Sharp/van der Heijde score = 0. Juxta-articular osteoporosis is a typical finding in hand X-ray in RA but rare in AS
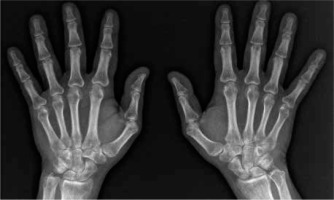
Table 1
Treatment modification and disease activity over time
Table 2
Comparison of both disease entities: criteria, symptoms, and treatment recommendations in relation to the described patient
[i] CRP – C-reactive protein, ESR – erythrocyte sedimentation rate, NSAIDs – non-steroidal anti-inflammatory drugs, AS – ankylosing spondylitis, TNFi – tumor necrosis factor inhibitors, IL – interleukin, csDMARD – classic synthetic disease-modifying antirheumatic drugs, JAKi – Janus kinase inhibitors
Discussion
According to previous reports, RA and AS rarely coexist in the same patient [2]. Rheumatoid arthritis is an erosive polyarthritis leading mainly to peripheral joint destruction (erosions, joint space narrowing) and AS predominantly affects the sacroiliac joints and spine with ankylosis and new bone formation (syndesmophytes) [3]. In the general population, RA and AS occur with a comparable frequency from 0.3% to 1.5%. Epidemiological data, as well as the higher incidence of both RA and AS in certain families and identical twins, support the significance of genetic factors [2]. HLA-B27 contributes to the risk of AS, while HLA-DR4 and DR1 are associated with RA [4]. The serological markers characteristic of RA include RF and ACPA autoantibodies. Their presence is one of the criteria for the classification as RA. These autoantibodies occur in 80% of cases of established RA [4]. In principle, RF in the IgM class should not be present in seronegative spondyloarthropathies including AS. RF may be occasionally detected in patients with AS, but generally in low titer and in IgA isotype. Current treatment of RA is based on early initiation of a classic synthetic disease-modifying antirheumatic drugs (csDMARDs) such as methotrexate, with (according to EULAR) or without systemic steroids (according to ACR). If it does not help, other DMARDs can be utilized and, if not effective, targeted synthetic DMARDs, such as JAK-inhibitors (JAKi) or biological DMARDs (bDMARDs), such as tumor necrosis factor inhibitors (TNFi), anti-IL-6 drugs such as tocilizumab, and B-cell depleting therapy with rituximab can be used to facilitate sustained remission and slow radiographic progression [5]. NSAIDs are the first line of AS therapy. Second-line drugs include TNFi, IL-17 inhibitors, and JAKi (tofacitinib and upadacitinib). Tumor necrosis factor inhibitors therapy has strikingly improved signs and symptoms as well as quality of life in more than two-thirds of AS patients with an inadequate response to NSAIDs [6]. Unlike RA, anti-IL-17 biologics, such as secukinumab, have proven to be beneficial in AS. Since the co-occurrence of RA and AS is rare, single case reports or case series have dominated over the years. A review of 81 cases of RA and AS comorbidity by Flores-Robles et al. showed that RA was the first disease diagnosed in 52% of the cases [7]. The drugs most used in the treatment of patients with AS and RA reported in recent works were glucocorticoids, methotrexate, NSAIDs and sulfasalazine [7, 8]. The data on use of biologics in RA and AS co-occurrence is scarce but there are case reports of patients treated with TNFi (etanercept) or with rituximab [7, 9, 10]. In our patient, due to the lack of effectiveness of TNFi (etanercept) and JAKi (tofacitinib), anti-IL-6 (tocilizumab) was used. The patients was reluctant to undergo 6-month immunosuppression; thus, rituximab was left as the last line of treatment after possible failure of TNFi, JAKi and IL-6i. Tocilizumab caused clinical improvement and allowed remission of both RA and AS. To our knowledge, it is the first case of seropositive RA that occurred in a patient with established AS on anti-IL-17 treatment that required a change for more specific treatment of RA (tocilizumab in this case). Despite the role of IL-17 in RA pathogenesis, blockade of this cytokine did not show efficacy in clinical trials [11], and it was also ineffective and even potentially pathogenic in inflammatory bowel disease [12]. It has been noted that secukinumab can induce a number of adverse events of special interest (AESI) 3 days to 96 weeks after secukinumab treatment. The four most common AESI were inflammatory bowel disease, eczematous drug eruption, drug-associated vasculitis, and drug-induced lupus erythematosus [13]. Cases of secukinumab-induced scleroderma, pustular psoriasis, and sarcoidosis have also been reported [14-16]. Secukinumab has the potential to develop several AESI by dysregulation of different expression of the polarity of T cells (Th1, Th2, Th17, Th22, and/or Treg) and reshaping the profile of cytokines in some patients [13]. It is widely accepted that cytokine blockade with bDMARDs can result in a variety of immune-mediated adverse effects including new onset or exacerbation of psoriasis despite its broad utilization in psoriasis treatment [17]. IL-6 seems to be an important cytokine that drives the production of IL-17. We can hypothesize that due to the specific cytokine endotype of this patient, inhibition of IL-6 with tocilizumab eventually allowed the control of the signs and symptoms of RA and AS. On the other hand, trials with anti-IL-6 treatment did not show clinical efficacy in treating AS and tocilizumab is not being positioned in management guidelines of AS [18]. Currently, no experimental data provide support for these findings; however, in a clinical setting, it is important to consider the possible coexistence of RA and AS in an individual during disease-specific target synthetic or biologic therapy, since paradoxically, an immunosuppressive agent can trigger the onset of other immune-mediated disease. Manifestations suggesting overlap of these two disease entities should prompt diagnostic work-up, new diagnosis and ultimately treatment modification possibly adjusted for cytokines engaged but with the priority of the clinical context.


