Nonsteroidal anti-inflammatory drugs (NSAID)-exacerbated respiratory disease (N-ERD) is defined as a chronic eosinophilic, inflammatory disorder of the respiratory tract, occurring in patients with asthma and/or chronic rhinosinusitis with nasal polyps. The symptoms become exacerbated by NSAIDs, including aspirin [1, 2]. This clinical syndrome was first described by Widal et al. [3] in 1922 and then revisited by Samter and Beers in 1968 [4]. In 2019, the panel of EAACI experts stated that the term N-ERD is more proper to describe the syndrome of respiratory hypersensitivity to NSAIDs and that previously used names like aspirin-exacerbated respiratory disease (AERD), aspirin-induced asthma, and aspirin triad should be abandoned. According to the experts, “NSAID” is a more inclusive term to replace “aspirin” in descriptions of this subtype of hypersensitivity to medications inhibiting cyclooxygenase [1].
N-ERD affects 0.3–0.9% of the general population, with much higher prevalence in asthmatics (10–20%), and even higher in asthmatics with nasal polyposis (30–40%) [5]. The onset of N-ERD is observed particularly in the third or fourth decade, although aspirin or NSAID sensitivity may develop at any stage of the disease process. The condition has a male predominance (2.3 : 1); however, when diagnosed in women, the disease is usually more severe [6]. Clinical features of N-ERD comprise initial nasal congestion with anosmia, with progression to chronic pansinusitis and nasal polyposis. Asthma may precede the upper airway disease or develop later (1–5 years) [7].
As observed in computed tomography (CT) scans of N-ERD patients, pansinusitis is some of the worst seen in chronic sinus disease, and complete or near-complete opacification of the sinuses takes place. Depending on the disease stage, surgery consists of polypectomy, functional endoscopic sinus surgery, or ethmoidectomy of particularly persistent cases – treatment with topical steroids is also necessary afterwards [6, 8]. Unfortunately, due to the progressive nature of the inflammatory process in N-ERD, surgery is unlikely to be curative, and even when followed by proper medical care patients require multiple revision surgeries during their lifetime. A history of having an asthma attack after ingestion of aspirin or other NSAIDs is highly suggestive and may be diagnostic. It is worth emphasising that there is no in vitro test that can be reliably used for the diagnosis of N-ERD. Therefore, the diagnosis is established on the basis of provocative challenge to either aspirin, lysine-aspirin, or another NSAID, which is considered the gold standard. There are four possible routes of aspirin provocation: oral, bronchial, nasal inhalation, and intravenous [1, 9].
In 1976, Zeiss and Lockey described a 72-hour refractory period after oral challenge in aspirin-sensitive patients. Since that first report, multiple literature data have suggested that desensitisation and daily administration of acetylsalicylic acid creates tolerance and significantly improves symptoms and quality of life [7]. Of particular importance, it also decreases the formation of nasal polyps and sinus infections, reduces the need for oral corticosteroids and sinus surgery, and improves nasal and asthma scores in N-ERD patients [10].
The aim of this report was to present cases of the first two N-ERD patients attending the program of aspirin desensitisation, which has been initiated in the Department of Otolaryngology and Laryngological Oncology, with the cooperation of the Department of Dermatology. The authors focused on the course, effectiveness, and side effects of the desensitisation process. Both patients presented with a positive oral aspirin challenge (performed during previous 3 days of hospitalisation) and then underwent desensitisation for 14 months.
Patient number 1 was a 52-year-old male who had suffered from N-ERD since 2010. He was diagnosed with asthma and rhinosinusitis with nasal polyposis. He underwent 2 functional endoscopic sinus surgeries (FESS) – the second FESS performed 4 months before desensitisation. He had a history of ASA and NSAID hypersensitivity, and in 2011 he suffered from severe broncho- and laryngospasm after taking an aspirin tablet. Concomitant diseases included hypertension and depression (Figure 1). Patient number 2 was a 41-year-old female, who had demonstrated N-ERD symptoms since 2005. She underwent 3 FESS (the last one was performed in 2014) and also had a history of chronic otitis media. Both patients were using nasal steroids chronically and were on a low salicylate diet. They both suffered from anosmia and lack of taste, which significantly influenced their quality of life. Table 1 presents the protocol of acetylsalicylic acid desensitisation used in our Departments.
Table 1
Aspirin desensitisation protocol used in the Department of Dermatology, Poznan University of Medical Sciences
| Day | Time | Dose of aspirin [mg] |
|---|---|---|
| 1 | 8.00 | 30 |
| 1 | 11.00 | 60 |
| 1 | 14.00 | 60 |
| 2 | 8.00 | 120 |
| 2 | 11.00 | 180 |
| 2 | 14.00 | 300 |
| 3 | 8.00 | 600 |
| 3 | 11.00 | 600 |
Figure 1
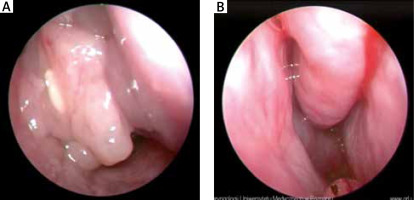
Endoscopic examination of the nasal cavity – patient number 1. A – Before treatment: polyps in nasal cavity and under middle turbinate. B – After treatment: nasal cavity is unobstructed and without polyps. Slight swelling and hyperplasia of the nasal mucosa
The chosen aspirin maintenance dose for both patients was 600 mg twice a day (1200 mg). The patients did not require any pharmacological treatment during the first 3 days of the procedure, which took place in the hospital in the controlled setting on the Dermatological Ward with the Intensive Care Unit available. Patient number 1 suffered from cough and headache on the first day, 1 h after ingesting 60 mg of aspirin. On the second day he reported headache and sneezing, which appeared 20 min after taking 120 mg of aspirin. The described symptoms lasted all day. On the last day of hospitalisation there were no complaints. Patient number 2 presented cough on the second day of desensitisation, 10 min after taking aspirin dose of 180 mg. The symptoms alleviated after 1 h and we continued the desensitisation procedure without any pharmacological support.
Daily aspirin therapy was well tolerated by both patients, and they attended periodic assessments, every month during the first 6 months of desensitisation and then every 2 months. Sinus endoscopy was performed every 3 months.
Most patients with N-ERD will benefit from aspirin desensitisation [5]. The treatment is particularly advised for patients with uncontrolled respiratory inflammation, who use high doses of corticosteroids to control the disease, and for patients with N-ERD, who require more than one sinus surgery [2]. Simon et al. [11] recommend surgical debulking of nasal polyps 2–4 weeks before aspirin desensitisation. The desensitisation procedure should also be advised for patients who need to be treated with aspirin for chronic disorders (cardiovascular diseases, arthritis) [5]. It is suggested that desensitisation and daily treatment with aspirin may increase quality of life and decrease the presence of nasal polyps, sinus infections, asthma attacks, and moreover reduces the need of corticosteroids and sinus surgery. The improvement can be observed after just 4 weeks of aspirin desensitisation [5]. In a study by Stevenson et al. [12] the authors observed an improvement concerning nasal symptoms in 25 patients who underwent aspirin desensitisation. A retrospective study by Ibrahim et al. [7] was carried out on 111 patients, being desensitised towards aspirin for approximately 12 months, with maintenance dose of 325 or 650 mg twice daily. Seventy-three percent of the patients claimed improvement of N-ERD symptoms and particularly appreciated the return of smell and/or taste. In a study presented by Spies et al. [13] the desensitisation procedure was accomplished by 8 out of 17 N-ERD patients. The initial dose was 1300 mg used for 6 months, and then decreased according to the clinical state for a consecutive 6 months. The authors emphasised that none of the patients followed after desensitisation required a new surgical intervention.
In the case of the described patients, we could observe improvement both in regard to their subjective and objective state (Figures 1 A, B). After 6 months of desensitisation process the patients did not need to use nasal corticosteroids and reported better control of concomitant asthma. Patient number 1 experienced an intermittent return of smell and taste. Patient number 2 noticed the return of smell on week 8 of desensitisation.
Although aspirin desensitisation is considered an effective method of treatment, the side-effects seem to limit its more widespread use. Stevenson et al. [2] created a list of six potential shock-organ responses during oral aspirin challenge: bronchospasm, laryngospasm, rhinitis/conjunctivitis, generalised urticaria, gastric pain, and hypotension. Lee et al. [5] described a group of 172 patients who underwent 1-year aspirin desensitisation (650 mg twice daily). Fourteen per cent of the group had to withdraw from the treatment due to the following side effects: gastric pain and bleeding, epistaxis, and urticaria. According to Simon et al. [11], the average rate of adverse reactions to aspirin therapy ranges from 8% to 23%. According to Ibrahim et al. [5], the most common unwanted reactions in desensitised patients included the following: gastrointestinal upset, easy bruising, tinnitus, gout, and hypertension. In our case series patient number 1 experienced gastric pain (relieved by increasing the dose of proton-pump inhibitors), and patient number 2 complained of ear blockage during first 3 weeks of desensitisation (alleviated with the use of local steroids).
To conclude, literature data and authors’ experience indicate that N-ERD patients need an effective and safe treatment. Aspirin desensitisation as a method of treatment seems to meet these conditions, and it can significantly improve the patient’s quality of life. Possible side-effects can be well controlled by specialists conducting desensitisation process. More research is needed on this complicated and multifactorial disorder.








