Introduction
The current European and American clinical guidelines for cardiovascular health coincide in the consensus that changes in lifestyles, such as maintaining healthy diet patterns, maintaining physical exercise, and quitting smoking, can help reduce the factors that can increase the risk of developing cardiovascular diseases [1]. Those changes in lifestyle are fundamental in menopausal women because the decrease in oestrogen levels associated with menopause increases the risk of cardiovascular events, as well as others such as long-term osteoporosis, representing the main cause of morbidity and mortality among menopausal women (only 26% in the UK) [2]. Vascular functions are modulated by oestrogen levels, with oestrogen receptors found in vascular smooth muscle, in endothelial cells, and mediating the release of prostacyclin and nitric oxide, both of which are powerful vasodilators. Other important risk factors in the development of cardiovascular diseases for menopausal women are the typical ones: age, weight, systolic blood pressure, glucose and cholesterol levels (total, low-density lipoprotein, and high-density lipoprotein), presence of hypertension, diabetes, and maintenance of the smoking habit [1]. Different studies connect body mass indexes above 25 together with habitual smoking, not only with a higher cardiovascular risk, but also with a higher risk of experiencing vasomotor symptoms [3]. Furthermore, the prevalence of the appearance of hypertension during menopause is twice as high as in premenopausal women, so maintaining blood pressure levels is highly necessary at this phase [2].
Due to of the above, the evaluation of the cardiovascular risks of women who normally smoke and who have reached the vital stage of menopause is highly important at this phase, due to the great impact that all these factors have on cardiovascular health and the development of future cardiovascular diseases.
Successfully quitting smoking is one of the healthiest lifestyle changes, although the objective is difficult to achieve [1]. The nicotine contained in cigarettes is one of the most consumed substances all over the world, and according to World Health Organization publications in 2021, there are more than 1.3 billion smokers in the world, resulting in one of the most widespread drugs of abuse [4]. Smoking is related to the appearance of a large quantity of diseases and to an increase in cardiovascular risk [2]. The harmful effects of smoking have been well documented. Diseases and other adverse health effects for which smoking is identified as a carcinogenic cause are bladder cancer, cervical cancer, oesophageal cancer, kidney cancer, laryngeal cancer, leukaemia, lung cancer, oral cancer, pancreatic cancer, and stomach cancer [5].
Currently, several smoking cessation services have been developed, both at the primary care level and in community chemist’s, all based on different guides such as “Supporting smoking cessation: A guide for health professionals’’, published by The Royal Australian College of General Practitioners, the aim of which is to serve as a guide for health professionals and patients in the smoking cessation process, collecting recommendations on the possible pharmacological treatments available, such as nicotine replacement therapy, varenicline, and bupoprion [6].
Cytisine has recently been added to available therapies for smoking cessation, although it is not new, having been used in Eastern Europe for many years. Its effectiveness must be evaluated for its inclusion in the guidelines for smoking cessation, while other sorts of therapeutic effects, which may be created, must be evaluated considering their use in menopausal women, so the purpose of this article is to summarize all the therapeutic actions and potential uses of cytisine in this population group. The review work of the articles was carried out during the period January–March 2022.
Material and methods
The review process was carried out following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [7]. The entire information search process began on 1 November 2021 and was carried out through an advanced search of databases such as Medline, PubMed (133 publications between 1950 present), Embase, Ovid, WOS, Proquest, and Scopus (period between 1988 and present), using as search terms “cytisine”, “smoking cessation”, “nicotine”, “women’s health”, and “menopause”. 1029 were found, excluding duplicates, of which 1022 were publications and 7 were articles in the press. Only articles written in English language were considered, although 14 articles were found in German, 9 in Polish, 6 in French, and 6 in Russian. The combined search of the chosen terms together with the term “menopause” did not produce any results in any of the databases. As selection criteria, those that described the characteristics and actions of cytisine, those that specified its use in the female, animal or human, gender were taken into consideration. Letters to the editor, non-clinical studies, and case reports were excluded. Table 1 shows randomized trials available in the last 10 years.
Table 1
Cytisine randomized trials in the last 10 years
Results
Cytisine
Cytisine plants of the genus Laburnum, family of Fabaceae, which includes species such as Laburnum anagyroides, Cytisus laburnum, or Laburnum alpinum among others, are characterized by the presence of the alkaloid cytisine, mainly in their seeds, in proportions sometimes greater than 5%, which are toxic [8]. Cytisine has traditionally been used in Eastern Europe as a respiratory stimulant, and during the Second World War it was used by soldiers as a substitute for tobacco due to its scarcity [9–11].
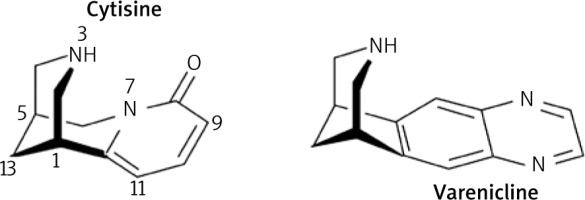
Cytisine has a three-dimensional structure, as shown in Figure 1, with an activity similar to that of nicotine, so that its target receptors will be nicotinic cholinergic receptors (AChRs) [12]. Activation of nicotinic (nAChR) and muscarinic (mAChR) AChR leads to effects in the different typical locations of these receptors, at the nervous, respiratory, urinary, digestive, cardiac, and blood vessel levels, in addition to the typical ones at the level of salivary, lacrimal, nasopharyngeal, sweat, and ocular secretion (miosis) [12].
Toxicity
The toxicity of cytisine has traditionally been associated with the consumption of the seeds that contain it (mainly Laburnum anagyroides, or Spartium junceum) [12].
Its effects in animals have been extensively studied, causing reactions linked to the toxic effects caused by nicotine [13]. In children and adults, studies have shown that cytisine intoxication can cause bowel movements, nausea, emesis, drowsiness, fatigue, dizziness, delirium, muscle twitching, and difficulty walking, all related to toxic nicotinic effects, resulting in reversible toxic reactions in most cases, with very few fatal cases [12]. The lethal dose in horses was established at 0.5 g/kg, while in humans it is considered safe at a unit dose of 4.5 mg, 3 times higher than the dose usually used in smoking cessation [12, 14].
Pharmacokinetics
The main pharmacokinetic characteristics of cytisine show that it is a very hydrophilic active ingredient in the protonated form and lipophilic in the basic form, being easily soluble in fluids such as gastrointestinal fluids, although its permeability at the gastrointestinal level is limited, both factors being possible causes of its low bioavailability [9, 12, 14–16]. The different studies in animals established a low penetration of the blood-brain barrier, 30% compared to 65%, which signifies a limitation to its clinical use compared to other derivatives of its structure, such as varenicline [12]. One recent cytisine meta-analysis found that the overall relative risk of successful continuous abstinence at the longest follow-up vs. placebo was 1.74 (95% CI: 1.38–2.19) [11, 14, 15].
Pharmacological action of importance in menopausal women
The different studies on cytisine have found different actions of this alkaloid, all related to its similarity to AChR. Among its possible uses in menopause, not only the option as a treatment in smoking cessation stands out, but also its possible mood regulation, cognition effects, weight, glycaemic, neuroprotective, cardiovascular, and anti-osteoclastogenesis effects, in which cytisine has shown activity in both animal and human studies. It is beyond the scope of this study to evaluate all aspects related to smoking cessation, so we will only take into consideration those that are related to or affect menopausal women.
Smoking cessation
Smoking is the most pernicious and dangerous habit in developed countries, being the main cause of mortality worldwide, with some 6 million deaths/year [9, 17]. The potential mortality of tobacco lies in the carcinogenic qualities of smoke and the presence of a heterogeneous amount of substances harmful to health [18]. The nicotine present in tobacco unleashes psychological and physical effects associated with its consumption, such as addiction and dependence, by interacting with mesolimbic nAChRs [12, 18]. Nicotine is not carcinogenic per se, but it does act as a facilitator of tumour growth produced by tobacco smoke, and with actions at the cardiovascular level, being toxic at the cardiac level to the doses used in smoking cessation, which can be an inconvenience in long-term smoking cessation treatments. In these cases, nicotine replacement therapy is replaced by other drugs such as cytisine or derivatives such as varenicline (Fig. 1) [12, 19].
Cytisine has been utilised effectively in smoking cessation since 1960 in Eastern and Central European countries (Tabex® Desmoxan®). Cytisine is a partial agonist of the α4β2 nAChR subtype and a full agonist of the α6β2 nAChR subtype, acting on the mesolimbic reward pathways, reducing withdrawal symptoms, and the pleasurable effects of nicotine [12, 19]. The affinity of cytisine for the α4β2 subtypes nAChR turn out to be 7 times greater than that of nicotine, although the activity of cytisine lies not in the activation of α4β2 nAChRs, but rather in their desensitization [9, 19].
Pre-clinical studies carried out in the last 40 years indicate that it has superior efficacy compared to placebo and to nicotine replacement therapy, even if they have been criticized for the methodology used [20]. None of the published studies have taken into account the aspects of their use in menopausal women, so the particular effects that may appear in this population group remain unknown.
Mood regulation and cognition effects
Cytisine has a similar activity to that of nicotine at the central nervous system level, but it requires higher doses due to its poor penetration of the BBB, and their effects are different. Cytisine decreases the effects of nicotine withdrawal, reducing the pleasurable effects of smoking, in addition to the dysphoric state caused by nicotine withdrawal [12]. Cytisine, by activating the nAChR subtypes α4β2 and α6β2, can modulate dopamine release, which may be responsible for the increase in cognitive activity, competing with nicotine for its linkage to nAChRs, minimizing the addictive effect of nicotine, and thus reducing nicotine withdrawal symptoms [12]. The ability of tobacco has been extensively studied in humans as well as in animals, showing antidepressant and anxiolytic effects due to the action of nicotine on nAChRs, decreasing cholinergic response [12]. Cytisine has shown efficacy in the control of depression and anxiety in animal models with acute and chronic depression, and, although its mechanism is not clear, it seems that the nicotinic and aminergic systems are involved, as well as action on serotonin reuptake, finding solid effects in clinical studies. Nevertheless, in human trials, this effect has been more limited, making it necessary to carry out a more complex type of study that considers its use in different types of depression [21, 22].
Body weight effects
One of the consequences of smoking cessation is weight gain in individuals who manage to quit smoking. The impact of nicotine on the weight of smokers is a widely studied phenomenon. Smoking reduces food intake and weight gain, and increases metabolism, so the cessation of its consumption makes ex-smokers return to their habitual weight [12]. Cytisine has been shown to be effective in reducing food intake in mice, in a similar way to nicotine. The exact mechanism of action is still unknown, although it appears to be related to the β4 subunit of the nAChRs of neurons of the arcuate nucleus of the hypothalamus [12].
The arcuate nucleus of the hypothalamus is a hypothalamic centre that regulates food intake, with neuronal populations that express different nAChRs with different subunits, including β4, whose stimulation or blockage causes the appearance of the sensation of hunger or satiety. The administration of cytisine in obese mice has been effective, while it has not been completely effective in smoking humans for long-term weight control, efficacy related to a lower activity in the β4 subtypes of nAChRs in humans [12].
Neuroprotective effects
Cytisine has shown in different studies its efficacy as a neuroprotective agent. Parkinson’s disease (PD) is a neurodegenerative disease in which the loss of nigrostriatal dopaminergic neurons causes a decrease in dopamine levels, leading to the appearance of cognitive and motor variations. Studies such as the one by Zarate et al. about the effects of the chronic treatment with cytisine in mice, brought to light the neuroprotective action of cytisine by reporting that a dose of 0.2 mg/kg improved both Parkinson’s behaviour and decreased the loss of dopaminergic neurons; these effects were only observed in female mice, and they were enhanced by the co-administration of 17-β-oestradiol [12]. Studies aimed at characterizing the possible neuroprotective effects of nicotine and its derivatives have found contradictory results. Some studies have correlated smoking with a certain protection of the development of PD, while other more recent and precise ones have found a relationship between smoking and an increased risk of developing Alzheimer’s disease as a result of increased cerebral oxidative stress and neuro-inflammation [12]. More studies are required to provide knowledge about these effects and their possible connection to other substances included in cigarettes.
Anti-tumoral effects
The cytotoxic activity of cytisine and its derivatives can act on many processes that surround tumours, such as apoptosis, and various targets and signalling pathways. The cytotoxic effect of cytisine has been observed inducing the apoptosis of tumour cells in human lung cancer and HepG2 human hepatocellular carcinoma cells; these are dose-dependent actions, which are characterized by causing mitochondrial dysfunctions and redox imbalances that lead to cell apoptosis. The continuing search for anticarcinogenic compounds leads to the development of new derivatives of cytisine, such as cytisine-N-methylene-(5,7-dihydroxy-4-methoxy)- isoflavone, a compound isolated from Sophora alopecuroides [7], traditionally used in China, which has revealed an inhibitory action of metastatic cells of breast cancer [23, 24]. More studies to deepen and characterize these tumour actions are required, being interesting, from the point of view of this work, for menopausal women.
Cardiovascular effects
The nAChRs have different locations at the sympathetic and parasympathetic level, not only peripherally but also centrally, thus being involved in cardiovascular control, and increasing blood pressure and heart rate [25]. Nicotine produces cardiovascular effects by sympathetic stimulation and the release of catecholamines after the activation of the nAChRs located in the peripheral sympathetic nerve endings and suprarenal medulla, causing increases in heart rate, blood pressure, and myocardial contractility [26]. Subcutaneous administration of cytisine in rats led to an increase in blood pressure similar to that obtained with the dosage of nicotine at low doses, as well as a reduction in heart rate that was not significant [12, 27]. The cardiotoxicity caused by chronic smoking may suggest the toxicity induced by nicotinic derivatives, because some studies such as the one by Wang et al. revealed myocardiotoxicity through experimentation with cardiomyocytes derived from pluripotential stem cells [28], so that the administration of cytisine at low doses (50 µM) has cytotoxic effects, increasing oxidative stress and calcium homeostasis, and causing functional changes in cardiomyocytes [28]. The cardiotoxicity of nicotinic derivatives makes it necessary to evaluate a proposal for the treatment for smoking cessation based on their safety.
Anti-osteoclastogenesis effects
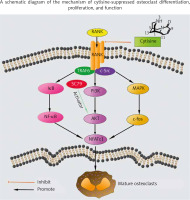
Osteoporosis is one of the long-term effects that occurs at the onset of menopause, where the loss of bone mass in women occurs in the 6 years after its onset, making its prevention and monitoring necessary. Among the risk factors associated with osteoporosis development and the appearance of fractures is smoking, which, together with the decrease in oestrogen levels at this phase, has a direct impact on the bone health of women [29]. Tobacco contains more than 4000 compounds, some of which have recognized osteoporotic effects, such as cadmium [20, 30, 31]. The effects of cadmium have been studied in the literature; even if its mechanism is not entirely known, it has been observed that exposure to cadmium even at low doses is related to the appearance of osteoporosis, osteomalacia, and increased risk of fractures [30–32]. Postmenopausal osteoporosis is characterized by an imbalance between the processes of osteogenesis and osteoclastogenesis. Cytisine has been shown to have the ability to decrease bone loss, as demonstrated by the study by Qian et al., which revealed the ability of cytisine to interact with macrophage colony-stimulating factor and receptor activator of NF-κB ligand (RANKL), suppressing RANKL-induced osteoclastogenesis, as displayed in Figure 2 [33]. The behaviour of cytisine in animal models [33] with induced oestrogen deficiency revealed an interesting anti-osteoporotic effect, which could be used in the treatment of menopausal women. More studies are required to characterize this activity of cytisine and the development of cytisine derivatives as promising medicines in this treatment [32].
A schematic diagram of the mechanism of cytisine-suppressed osteoclast differentiation, proliferation, and function
Conclusions
Cytisine is an alkaloid widely known and used for a long time, which has demonstrated its efficacy and safety in smoking cessation. It does not present relevant adverse effects (normally headache, dry mouth, or nausea), and it can be considered for use as a smoking cessation therapy of choice for menopausal women who are unable or unwilling to benefit from the use of varenicline, bupoprion, or nicotine replacement therapy. From the point of view of its recommendation and use in menopausal women, the impact of cytisine on smoking cessation in these women is not only limited to this, but also it can bring benefits at other levels. Cytisine can improve the cardiovascular health of the menopausal woman, whose risk increases during this period for major depression and anxiety, typical symptoms that can appear in the pre- and postmenopause, with models that improve cognitive capacity and the evolution of both major depression and anxiety. Further investigations are needed to investigate the effects that cytisine and its derivatives can provide. The antiosteoporotic activity that cytisine has shown is very promising, because its treatment and monitoring is essential in menopausal women, although further studies are required to characterize the behaviour of cytisine and its derivatives, which makes it highly eligible as an alternative in menopause. Cytisine is characterized by a certain cytotoxic action, an effect that has been used in the treatment of different types of cancer, such as lung, liver, or breast cancer. Currently, cytisine derivatives are under development, such as cytisine-N-methylene-(5,7-dihydroxy-4-methoxy)-isoflavone, with promising results in inhibiting the growth of breast cancer tumour cells; further studies are required to determine the scope of these effects with greater knowledge.
From the point of view of weight gain, cytisine has not been effective in the long term, although it has a limited impact in the short term, so the probable weight gain as a consequence of smoking cessation must be prevented at the beginning of the treatment by promoting measures to limit it, such as nutritional advice or the promotion of a healthy lifestyle and nutrition. Its authorization for use in smoking cessation supposes the addition of a new effective therapeutic tool for its treatment, which is also economical, so that its use can be cost-effective because the benefit for health in the prevention of future smoking-related diseases is favourable, with the consequent future savings that could be reported in the different health systems [34].