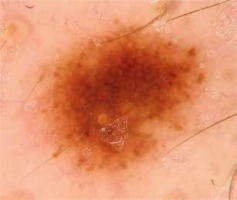
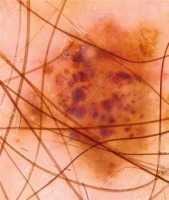
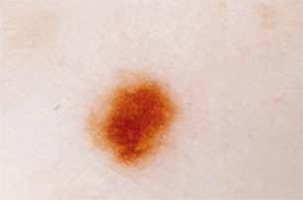
Introduction
Unless diagnosed and excised at an early stage, cutaneous melanoma (CM) is a potentially lethal malignant tumor [1–3]. In recent years, it has become a major public health challenge due to its increasing incidence and mortality [1, 4–7]. Positive family and personal history of melanoma, Fitzpatrick phototype I and II, UVR exposure and high total nevus count are well-known risk factors for development of malignant melanoma [8, 9]. Presence of plural nevi is both an independent predictor for melanoma and a potential precursor for the discussed malignancy [9–13]. Dermoscopy is commonly used in the evaluation of melanocytic nevi and in early detection of melanomas (Figure 1). An experienced dermoscopist identifies numerous dermoscopic structures and patterns in the examination. Dermoscopic inspection of specific patterns is conducted in order to differentiate between benign and potentially malignant lesions. Thus understanding the possible relation between the nevus pattern and melanoma development may be important for improving early detection of melanoma. Since the late 1970s “dysplastic” nevi have been known to be associated with increased risk of melanoma, especially if the patient has been diagnosed with dysplastic nevus syndrome (B-K mole syndrome). Unfortunately, nowadays the terms dysplastic nevus (DN) and dysplastic nevus syndrome are controversial, especially with regards to the histologic definition of DN and common lack of its correlation with the clinical picture [14–16]. What is more, there is insufficient research into the relation between dominant dermoscopic patterns including the patterns which are considered benign and the incidence of cutaneous melanoma.
Aim
The aim of the study was to determine if there is a predominance of one dermoscopic pattern in patients with melanoma and if there is a significant difference in the dominant global dermoscopic pattern in patients with cutaneous melanomas correlated with patients’ age, sex, and the location of the primary tumor.
Material and methods
This observational retrospective study was conducted by 2 dermatologists. It involved collecting videodermoscopic pictures and patient data from the Dermatology Department, Jagiellonian University Medical College in Krakow, Poland from the period 2013–2018. The study included 162 patients with a prior diagnosis of cutaneous melanoma. All the patients remain under observation at the Videodermoscopy Practice at the Dermatology Department, which is designated for the follow-up of patients from melanoma risk groups including patients with dysplastic nevus syndrome, neurocutaneous melanosis, positive personal and family history of melanoma. At the practice all patients diagnosed with melanoma underwent two total body dermoscopies conducted using Heine Delta/Delta Plus handheld dermoscopes (Heine Optotechnik). All examinations were conducted by two dermatologists. The first examination (total body dermoscopy and videodermoscopy of atypical lesions) was within 1 month since the diagnosis of cutaneous melanoma, and subsequent examinations were within 3–6 months (also total body dermoscopy and videodermoscopy of atypical lesions). During the examinations the global dermoscopic pattern was evaluated and atypical lesions were marked in order to be archived by means of videodermoscopy. The nevi were subclassified with regard to the pattern: reticular (Figure 2), globular (Figure 3), homogeneous and mixed pattern (two-component pattern; reticular-globular pattern) (Figure 4) with central or peripheral globules and multicomponent (mixed – at least 3 types of structures in one nevus). We defined the global pattern as dominant if it was seen in 50% or more of patients’ nevi. Melanocytic lesions meeting the criteria of atypical nevus (marked during dermoscopic examination) were reexamined using a videodermoscope and all acquired pictures (both micro- and macroscopic) were archived. The criteria for videodermoscopic monitoring of the lesion included asymmetry in 2 axes, border irregularity, color variability, abrupt peripheral cut-off of pigment network, asymmetrical/peripheral dots/globules [17–19]. Videodermoscopic pictures were acquired using videodermoscopes (Fotofinder, TeachScreen GmbH, Bad Birnbach, Germany) at minimum 20× and maximum 120× magnification.
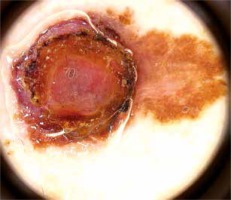
Figure 4
Junctional melanocytic nevus presenting mixed (reticular-globular) pattern. Lesion located on trunk
Statistical analysis
All acquired data were tabulated and all analyses were performed with Microsoft Excel (Microsoft Office 2019; Microsoft) and Statistica v 13.3 (StatSoft; TIBCO Software Inc.). The χ2 test was used to evaluate the association between dominant global pattern of nevi and patients’ sex/location of primary tumors (with a p-value of less than 0.05 considered statistically significant).
Results
The group consisted of 162 patients – 92 women and 70 men, aged between 26 and 97 years on the day of diagnosis (average 63 years, median 64 years, including 2 patients ≤ 30 years). The mean number of nevi in the patients was 97. Assessing the location of primary tumor: 45.06% (73) of the primary lesions were located on the trunk, 21.6% (35) on lower libs, 17.3% (28) on upper limbs and 16% (26) on the head (scalp). Taking into account skin phototype, all examined patients in the group were Fitzpatrick skin type II.
In 29.6% (48) patients’ melanomas were histologically assessed as in situ and 70.4% (114) were invasive. As assessed in the pathology examination, ulceration was present in 11 (6.79%) cases, while 151 (93.21%) cases did not involve any microscopically detectable ulceration. In 119 (73.5%) patients the mitotic index was 0/mm2 and in 43 (26.5%) cases the mitotic index was ≥ 1/mm2 up to 20/mm2.
In all patients global dermoscopic patterns were evaluated and subclassified as reticular (27.16%, 44 patients), globular (0%, no patients with dominant globular pattern), homogeneous (27.16, 44 patients), mixed (12.96%, 21 patients) and multicomponent (32.72%, 53 patients).
We performed the χ2 test to assess the relation between prevalence of each subtype of global dermoscopic patterns and patients’ sex (Table 1). The reticular pattern was significantly more prevalent in male patients (38.57%, 27 patients) in comparison to female patients (18.45%, 17 patients) (p = 0.041). There was no significant predominance of other patterns depending on patients’ sex.
Table 1
Relationship between global pattern of nevi and sex in patients with cutaneous melanoma. Various indexes (a and b) show statistically significant differences
In terms of a relation between dominant global pattern of nevi in patients and the location of primary tumors using the χ2 test we found a statistically significant (p = 0.030) lower prevalence of the reticular pattern in patients diagnosed with melanomas located on upper limbs. The homogeneous pattern was statistically significantly more prevalent in patients in whom primary tumors were located on the head and upper limbs.
Discussion
Previous studies have shown an increased risk of melanoma in patients with a high nevus count or plural dysplastic nevi. A dysplastic nevus is defined as a clinically atypical nevus usually when it exceeds 5 mm in diameter, has variable pigmentation and poorly defined borders. According to the definition DN shows melanocytic dysplasia in pathological examination. However, the concordance between clinical and histological diagnosis of dysplastic nevus is rather low. In the discussed study we evaluated all melanocytic lesions by means of dermoscopy, and melanocytic nevi meeting the criteria of atypical nevus (asymmetry in 2 axes, border irregularity, color variability, abrupt peripheral cut-off of pigment network, asymmetrical/peripheral dots/globules) were reexamined using a videodermoscope. In all examined patients the dominant global dermoscopic pattern was evaluated twice (during two subsequent tests) and classified as reticular, globular, homogeneous, mixed and multicomponent.
Literature data suggest that the prevalent nevus pattern may be influenced by age, sex, Fitzpatrick’s skin type, UVR exposure and history of melanoma.
UVR exposure – a well-known risk factor for development of CM – has been proven to increase the prevalence of acquired melanocytic nevi [20], and the number of large (> 5 mm) acquired melanocytic nevi is proportional to the number of sunburns [21]. Nevi exposed to UV radiation show darkening of pigmentation, fading of pigment network, increase in size, development of irregular dots and globules – though changes are described as reversible [22, 23].
In our study there were no patients showing a globular pattern of nevi as the predominant one. The examined patients presented with every other pattern of nevi as the predominant one with various frequency (reticular 27.16%, homogeneous 27.16%, mixed 12.96%, and multicomponent 32.72%). In the study conducted by Zalaudek et al. globular nevi were significantly more prevalent in people between 2 to 20 years of age and the prevalence of such nevi apparently decreased with patients’ age to reach 0.9% in the population aged > 75 years [24]. The Framingham school nevus study conducted by Scope et al. also showed the globular pattern as the predominant one in 38% of examined school children [25]. Douglas et al. researched dermoscopic nevus patterns in people at high versus moderate/low melanoma risk and also found that globular nevi are more commonly observed in younger groups [26]. In our study patients’ age varied between 26 and 97 with an average of 63 and median of 64 years, which suggests that most patients were at the age which is less susceptible to plural globular nevi in comparison to the younger population.
The prevalence of any atypical nevi in white-skinned populations ranges from 30 to 60 percent of patients with a personal history of melanoma in comparison to 2–10% in the white population with no history of melanoma [27, 28]. Due to the well-known correlation between the presence of atypical nevi and increased risk of melanoma, digital dermoscopy is commonly recommended in people with plural atypical nevi.
The focus of our study was the relationship between the predominant pattern, patients’ sex and location of the primary tumor. We found no subgroup of patients (previously diagnosed with cutaneous melanomas) with statistically higher presence of the dominant global multicomponent or mixed (2-component) pattern – which are clinically and dermoscopically most consistent with atypical/dysplastic nevus.
The research by Lipoff et al. into the global pattern of nevi on back skin revealed that nevi with the complex dermoscopic pattern are more prevalent in patients who develop melanoma. In the cited study the group of researchers considered all nevi presenting both a pigment network and globules (with or without structureless areas) complex. The study, however, had some limitations including a small sample group and the fact that only the nevi on the patients’ backs were evaluated. In our study we classified the nevi described by Lipoff as complex either as mixed (2-component) or multicomponent. The predominant multicomponent pattern was most prevalent (32.72 %) and together with the mixed pattern occurred in 45.68% of all cases.
Duffy et al. in their study examining the relationship between subtype counts of nevi (without determining the dominant subtype) and cutaneous melanoma found that the number of homogeneous subtype nevi was most highly associated with CM risk, followed by the complex pattern, then reticular then globular [29].
On the other hand, we found that the dominant reticular pattern is significantly more prevalent in male patients (38.57%) with melanoma in comparison to female patients (18.45%) (Table 1). Since the reticular pattern is commonly considered benign in comparison to the multicomponent pattern, we find the result inconsistent with previous studies, but we regard this result an important stimulus for further research in this area, especially considering the fact that melanomas diagnosed in male patients in the study group showed a statistically significantly higher Breslow’s index value (0.16 mm) in comparison to female patients (0.12 mm).
In an attempt to assess a possible relation between a dominant global pattern of nevi in patients and location of primary tumors we found statistically significantly lower prevalence of the reticular pattern in patients diagnosed with melanomas located on upper limbs (Table 2). The homogeneous pattern was statistically significantly more prevalent in patients in whom primary tumors were located on the head and upper limbs. There was no group of patients with a specific location of tumors that would present with a dominant complex or multicomponent pattern.
Table 2
Comparison of the location of the primary tumor with prevalence of global pattern in patients. Various indexes (a and b) show statistically significant differences
The limitation of the study is that the dermoscopic pattern of melanomas was not evaluated, since not all melanomas were evaluated by both researching dermatologists (some of the patients were referred to our clinic after primary resection of the tumor – therefore dermoscopy/videodermoscopy of the tumor was not performed). However, we have conducted and published such an evaluation (dermoscopic features in different dermatopathological stages of cutaneous melanomas) in 81 of 162 described patients [30].
Conclusions
Our study suggests that predominant complex patterns are more commonly observed in patients diagnosed with cutaneous melanoma. On the other hand, there is a significant number of melanoma patients with predominant reticular and homogeneous patterns, which are not often recognized as associated with an increased risk of development of melanoma.
In the light of our research and increasing incidence of cutaneous melanoma, we believe that we may not disregard screening in patients with a seemingly benign dominant pattern of nevi, and the utility of predominant dermoscopic patterns for predicting melanoma risk shall be studied further.