Introduction
Juvenile systemic lupus erythematosus (jSLE) is an auto- immune disease characterized by its onset in childhood or adolescence, high immune activity, and a wide spectrum of clinical symptoms [1-3]. Its etiopathogenesis involves many mechanisms, such as dysregulation of apoptosis, production of autoantibodies, formation of immune complexes, and activation of the complement system [4, 5]. The heterogeneity of these mechanisms explains the variety of the clinical symptoms as well as the spectrum of the responses to jSLE treatment [6]. In recent years, activation of the interferon (IFN) pathway has gained interest, as it causes chronic, uncontrolled stimulation of the immune response [5, 7, 8], which is also observed in nearly 50% of adults and the vast majority of children diagnosed with SLE [8]. One of the early and severe manifestations of jSLE is lupus nephropathy (LN), which eventually develops in about 90% of cases [9]. Evidence shows that the IFN pathway is involved in the early, preclinical phase of LN development [5].
Nephropathy is the first manifestation of systemic lupus in about 50-60% of pediatric patients. However, approximately 25% of children do not meet the diagnostic criteria for lupus at the time of the first symptoms of nephropathy [1]. Early diagnosis of LN is crucial for further disease management, as kidney dysfunction contributes to the increase in lupus mortality and, in about 19% of cases, to the development of end-stage renal disease [1, 5]. The clinical manifestation of nephropathy varies from asymptomatic hematuria, mild proteinuria, nephrotic syndrome, nephritic syndrome, thrombotic microangiopathy, rapidly progressive glomerulonephritis, to acute or chronic renal failure [1, 9]. The clinical picture of nephropathy does not correlate with the type and intensity of histopathological changes and does not predict its course. The basis for LN diagnosis is a renal biopsy, which allows one to categorize and assess the level of activity and chronicity of the disease process [1, 5, 9]. Characteristic histopathological changes in biopsy may considerably anticipate abnormalities in urine tests. According to the American College of Rheumatology (ACR) recommendation from 2012, biopsy should be performed in patients with active SLE when kidney involvement is suspected, as well as in children with isolated proteinuria and the presence of ANA and dsDNA antinuclear antibodies [9, 10]. However, approximately 10% of pediatric LN patients do not have auto- antibodies, which does not rule out LN development [11].
The biopsy evaluation should include light microscopy, immunofluorescence, and transmission electron microscopy (TEM) [10]. TEM examination is used to assess the basement membrane, the morphology of pod- ocytes, and the presence and location of deposits [12]. It also makes it possible to reveal the presence of specific intracellular structures such as tubuloreticular inclusions (TRIs) [13, 14].
Tubuloreticular inclusions are visible as delicate reticular aggregates of branching membranous tubules located near or within the endoplasmic reticulum cisterns [15-17]. TRIs were first detected in endothelial cells, but they also have been observed in monocytes, lymphocytes, Schwann cells, and fibroblasts [13]. In samples collected from SLE patients, TRIs were observed first 50 years ago in the glomerular endothelium and were originally termed lupus inclusions [13]. So far, their meaning has not been fully defined. The ultra-cytochemical studies by Rich et al. have shown that TRIs consist of proteins, lipids, and RNA [14, 18]. They have been observed in cases of viral infections, especially human immunodeficiency virus (HIV), cytomegalovirus (CMV), hepatitis B virus (HBV), and Epstein -Barr virus (EBV) infections, as well as after IFN treatment and renal transplants [13, 16, 19, 20]. Recently, numerous TRIs have been reported in endothelial cells of the glomerular capillaries in the course of COVID-19 infection [21]. Viral infections are the second most common group of diseases in which TRIs are commonly seen. The common link between nephropathy induced by a viral infection and LN is IFN-α [19].
Nephropathy and TRIs’ presence often precede other symptoms of SLE many months earlier, which is why TRIs have become a recognized and useful pathomorphological marker of LN in cases of any diagnostic doubts [10]. Such a situation occurs mainly in pediatric cases in which the symptoms are often uncharacteristic and the final diagnosis is hard to make. Unfortunately, there are not many studies focusing on TRIs in jSLE. This study aims to evaluate renal biopsies of 10 patients with jSLE for presence of tubuloreticular inclusions. It also aims to assess TRIs’ occurrence in relation to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) lupus nephropathy classification, SLE clinical manifestation, and serological test results.
Material and methods
It is a preliminary study based on a small number of patients. The study was conducted in compliance with the Declaration of Helsinki. The Ethics Committee of the Medical University of Silesia approved (PCN/0022/KB/46/21, 19th February 2021) of the use of the retrospective data from patients’ files. Signed informed consent forms were obtained from all ten patients and their parents.
Analysis of clinical data
A retrospective analysis of the medical records of 10 pediatric patients diagnosed with jSLE and LN was performed. The following data were analyzed: the clinical course of the disease, the manifestation of nephropathy, the profile of non-renal symptoms (including hematological disorders), and the immune activity of lupus. Lupus activity was assessed according to the ACR (American College of Rheumatology) and EULAR (European League Against Rheumatism) scale.
Renal biopsy samples’ analysis
Renal tissue material obtained by needle biopsy of the studied patients was analyzed. Routine histopathological examination of collected samples was performed and based on the results of the examination they were assigned to the LN class according to the ISN/RPS nephropathy classification.
For light microscopy, semi-thin sections (1 µm) were cut on a glass knife and stained with toluidine blue to find a region of interest.
For TEM analysis, renal biopsy samples were prepared according to the conventional procedure. Ultrathin sections (70 nm) were cut from representative samples with a diamond knife (45°; RMC, Tucson, USA) using a POWER TOMO PC ultramicrotome (RMC Boeckeler, Tuscon, USA), mounted on 300-mesh copper grids, and stained with 0.5% aqueous uranyl acetate and lead citrate (LAURYLAB, Saint-Fons, France) using a Leica EM AC 20 stainer (Leica Microsystems, Vienna, Austria). The grids were air-dried and examined later in a TECNAI G2 12 Spirit BioTWIN transmission electron microscope (FEI, Eindhoven, The Netherlands) at 120 kV.
The images from representative regions were taken with a Morada CCD camera (Olympus Soft Imaging System Solutions GMBH, Münster, Germany) and the ultra- structure of the glomeruli was assessed. Obtained images were analyzed for the location, structure, and size of the deposits, changes in podocytes and the basement membrane, and the presence or absence of TRIs in the analyzed material. Other pathological changes at the ultrastructural level were also examined. The results of the assessment were compared with the clinical presentation, laboratory test results, and the ISN/RPS nephropathy classification.
Literature review
PubMed, Scopus, and Embase were used to search relevant articles published from 1980 to 2020. The search terms used were ‘pediatric’ AND ‘lupus’ AND ‘tubuloreticular inclusions’. Any original research articles, written in English and published in peer-reviewed journals were eligible for initial literature review. All articles included in the evaluation needed to have used keywords in their abstract or title. The search identified 7 studies with 5 articles meeting the above-mentioned inclusion criteria.
Results
All ten patients enrolled in the study group were female. In three cases, the onset of the disease occurred before the age of ten, in seven cases in adolescence. All patients enrolled in the study group met the criteria for SLE with clinically developed nephropathy. According to the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), they received from 16 to 47 points (Table 1). Lupus nephropathy was assessed using the ISN/RPS classification.
Table 1
Variables describing patients with juvenile systemic lupus erythematosus (jSLE) enrolled in the study (n = 10)
Joint pain and swelling in nine cases and maculo- papular rash (including butterfly-shaped erythema) in four cases were the first clinical signs. Also, low-grade fever and neuropsychiatric symptoms were observed. In three patients, all of them being under the age of 10, the disease started with hematological disorders such as leukopenia, thrombocytopenia, and anemia (Table 1). In one case, the diagnosis of lupus was made in the coexistence of severe paranoid schizophrenia symptoms. Symptoms of nephropathy were observed in all patients starting from the onset of the disease. All ten patients developed proteinuria, some of them hematuria (n = 6), and sterile leukocyturia (n = 3) (Table 1). However, all patients presented a normal glomerular filtration rate (GFR). All patients showed high immunological activity of SLE – the ANA antinuclear antibody titer exceeded 1 : 640. We also noted a high anti-dsDNA titer and low values of the complement components (Table 2). In our study group, all children have been receiving hydroxychloroquine (200 mg/day) since the lupus diagnosis.
Table 2
Results of immunological tests of patients with juvenile systemic lupus erythematosus (jSLE) enrolled in the study (n = 10)
The evaluation of the biopsy samples in a light microscope revealed class III LN in five cases (50%), class II in three cases (30%), and class IV and class V in two individual cases (Table 1). Ultrastructural analysis of the biopsy material showed the presence of TRIs in seven patients (Table 3). These structures were most often found in endothelial cells of both the vascular bundle and the peritubular capillaries. They were occasionally observed in monocytes within capillaries and mesangial fields (Fig. 1). TRIs occurred within the irregularly widened cisterns of the endoplasmic reticulum. Some of them presented signs of pronounced swelling, which led to disruption of the surrounding membranes. In some inclusions, the tubular structures came into close contact with the membranes of the endoplasmic reticulum (Fig. 2). Both the size of the inclusions and the frequency of their occurrence varied among specimens (Fig. 3). The size of the TRIs varied in the range 300-1600 nm. Numerous TRIs (several inclusions in one glomerulus) were found in the biopsy of a patient with class V LN. All patients were tested for the most common viral infections connected with presence of TRIs (HIV, HBV, HCV, EBV, CMV) and all those disorders were excluded by performing simple blood tests. Only in one patient, after three years of treatment, was the re-biopsy performed. TRIs were still present in the specimen, but they were much less abundant (Fig. 4). In three cases with the highest presence of TRIs, the disease began before puberty (at 8-10 years of age), and in all these cases hematological disorders such as leukopenia, anemia, and thrombocytopenia preceded the LN diagnosis. Inclusions in a typical location, near subendothelial deposits, were observed in only one patient (Fig. 5). Biopsies of three patients with disease onset in adolescence (around 14 years of age) revealed no TRIs in the sampled material. In these patients, arthritis and nephrotic proteinuria dominated the clinical picture.
Table 3
Results of transmission electron microscope (TEM) evaluation of renal biopsy samples of patients with juvenile systemic lupus erythematosus (jSLE) enrolled in the study (n = 10)
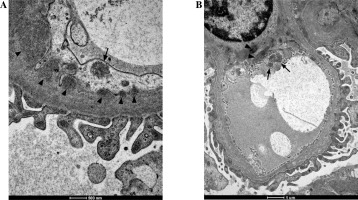
Fig. 1
Tubuloreticular inclusions (TRIs) observed in monocytes (A) and macrophages (B) in renal biopsy samples collected from patients with juvenile systemic lupus erythematosus (jSLE). TRIs are indicated with arrows
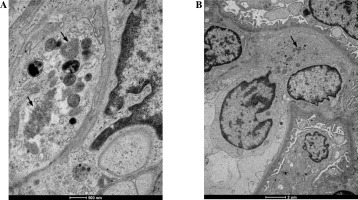
Fig. 2
A, B) Evident tubuloreticular inclusions (TRIs) visible within the degranulated rough endoplasmic reticulum in renal biopsy samples collected from patients with juvenile systemic lupus erythematosus (jSLE). Full arrowheads indicate loss of the reticular membrane continuity. Open arrowheads indicate adherence of individual tubules to the membranes of the reticulum
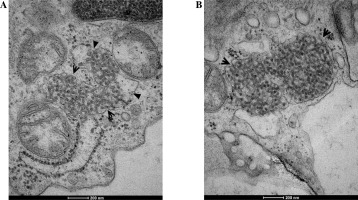
Fig. 3
Fragment of a capillary with a highly swollen endothelial appendix containing three tubuloreticular inclusions (TRIs) (arrows) in renal biopsy samples collected from patients with juvenile systemic lupus erythematosus (jSLE)
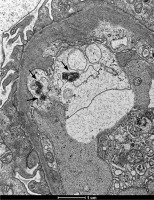
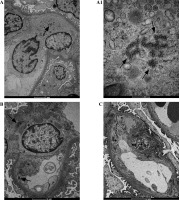
Fig. 4
The figure shows two biopsies of Patient 6. The first biopsy was performed in 2012: A) TRI structures (arrows) are present in a monocyte, B) endothelial cells and within mesangial area; A1) Enlargement of the photo (A) revealing details of the ultrastructure of TRI structures. The second biopsy (C) performed 3 years after initiation of treatment revealed absence of TRIs
Discussion
The attempt to explain the importance of TRIs in LN has a long history, but their diagnostic, pathophysiological, and prognostic role has not been clearly defined so far [13, 17]. The presence of TRIs in the endothelial cells of the glomerular capillaries is considered a very important morphological marker of LN, especially in pediatric cases [11, 16, 22]. TRIs are sometimes called ‘interferon footprints’ because they are considered markers of the IFN pathway [13]. The amount of TRIs is linearly dependent on IFN production [7, 17].
Our ultrastructural studies of renal biopsies in children with jSLE and LN showed TRIs’ presence in 7 out of 10 patients, which is consistent with the observations of other authors in the adult population [14]. Unfortunately, there are not many studies concerning TRIs in children suffering from jSLE. In the pediatric population, the first report of TRIs’ presence in an ultrastructural study of renal biopsies concerned non-LN-related cases. The report described children with severe nephrotic syndrome and rapid progression to end-stage renal disease (ESRD) in whom HIV, CMV, and HCV infections were excluded. The authors suggested that the formation of TRIs resulted from the IFN pathway activation signaling the distorted immune response to common viral infections (rhinoviruses, enteroviruses, coronaviruses) [23].
Interferon is a common pathophysiological link between LN and viral nephropathy [23, 24]. IFN mediates most of the symptoms and pathophysiological processes underlying lupus. IFN also has a suppressive effect on the bone marrow which may result in anemia, leukopenia, and thrombocytopenia [4, 7, 8, 17]. We observed numerous TRIs in endothelial cells in renal biopsies of the youngest patients with prepubertal onset of the disease. These patients developed severe hematological disorders such as leukopenia, thrombocytopenia, and anemia in the early phase of the disease. Blocking the biological activity of IFN-α can stop the inflammatory process, alleviate autoimmune activity, and protect against onset of flares [4, 5]. Anifrolumab, a monoclonal antibody against the IFN-α type 1 receptor, has been under clinical trials. The research has not covered the cases of LN, only the cases of SLE with non-renal manifestation. The authors announced the continuation of the study in LN, which raises new hopes for early, targeted therapy in children with jSLE with kidney involvement [5].
In their work, Tranesh [25] suggested a relationship between the number of TRIs and the nature of histopathological changes in glomeruli, which are the basis for the LN classification. The author showed that the number of TRIs correlated with the ISN/RPS LN class: the increased number of TRIs correlated with class III, IV (proliferative forms) and V (membranous glomerulonephritis – MGN). In cases of MGN, the formation of TRIs was found in 100% of cases [24]. In our study, class V LN was diagnosed only in one patient. We found numerous clusters of TRIs in the ultrastructural image of this patient’s renal biopsy. In the re-biopsy performed after 3-year treatment resulting in remission, these inclusions were still present, but less abundant. In our study group, the proliferative forms of LN dominated, which is typical of jSLE. Due to the small size of the study group, it was impossible to correlate the amount of TRIs in the biopsy with the LN class.
Testing for TRIs is a valuable clue in cases where the diagnosis of SLE is not fully established and the clinical manifestation of lupus is nonspecific, which is often seen in pediatric cases [11, 16, 22]. An interesting example of the role of TRIs in making a final diagnosis is the case of a patient whose first and only manifestation of developing lupus was a severe drug-resistant depressive disorder. In this patient, additional assessment of the biopsy in TEM confirmed the diagnosis of lupus and made it possible to implement an effective treatment [26]. Bromfield et al. presented a case of an infant with severe nephrotic syndrome, anemia, and thrombocytopenia. The patient’s biopsy image indicated class V LN with the presence of numerous TRIs. The image was interpreted as a genetically determined disturbance in the IFN pathway [13]. According to the Mayo Clinic recommendations, TEM examination should be permanently included in the generally applicable guidelines for assessment of renal biopsies in LN [12].
A significant limitation of this study is the small number of pediatric LN patients and the biopsy samples we assessed in TEM. The small number of samples limits the possibility to accurately assess the relationship between presence of TRIs, symptoms, and serological indicators of the disease. That is why it is a preliminary study based on a small number of patients and we are going to continue our research. Moreover, the ultrastructural assessment is limited to a small number of glomeruli present in the samples. Since the formation of TRIs does not affect all endothelial cells, the number of inclusions in the image of a single glomerulus can vary greatly. They can be absent in the image of one glomerulus, while being present in the image of different glomeruli of the same patient. Furthermore, the number of visualized TRIs closely depends on the cross-section made during the preparation of the tissue material. All of these factors make it necessary to proceed with great caution when assessing the intensity of TRIs’ formation, their number, and size. Another limitation of this study is that the biopsies have been performed at a different time since the onset of the disease and we know that the formation of inclusions varies in different phases of the disease.
Nevertheless, TEM images of renal biopsies in patients with jSLE are sources of inspiring information on the pathophysiological processes in renal cells. Based on available reports and our study, it can be concluded that the presence of TRIs in ultrastructural images of renal biopsies is a valuable pathophysiological clue and a sign of IFN pathway involvement in the autoimmune process at the earliest stage of the disease. The evaluation of renal biopsies in TEM should be an obligatory element of the diagnostic process in children with jSLE. It facilitates confirmation of the diagnosis, especially in the youngest patients and cases that do not meet SLE criteria.