Summary
The OCEANUS study confirmed the safety and efficacy of the MER™ stent during 30-day and 1-year follow-up in both symptomatic and asymptomatic patients. The majority of patients were event-free. However, larger cohort studies are needed to evaluate MER™ stents in detail.
Introduction
Carotid artery stenting (CAS) with distal and proximal cerebral protection devices is an established method of invasive treatment of atherosclerotic stenosis of carotid arteries. The most important randomised controlled trial, the CREST trial, demonstrated similar immediate and long-term outcomes of CAS and carotid endarterectomy (CEA) [1, 2].
Conditions that determine a good result of CAS include operator experience, as well as the proper selection of stents and protection devices based on the patient’s clinical condition, atherosclerotic plaque morphology, and the anatomy of the carotid artery [3–5].
Two types of self-expanding stents are used in conventional CAS: open-cell and closed-cell. However, balloon-expandable metal stents are preferred for the treatment of ostial common carotid artery (CCA) stenosis and brachiocephalic trunk stenosis. Another option is the use of so-called mesh-covered stents, which may be a solution to eliminate late embolic complications after CAS [6, 7]. However, these stents still require further long-term evaluation. The continuous progress and reduction of periprocedural complications are possible thanks to the use of new types of protection devices and stents. Importantly, centres performing CAS should be equipped with stents of different designs because the use of only one stent type and one protection device type (usually filter) in all treated patients is not a recommended strategy [3–5].
The criteria of an acceptable complication rate (a composite of death, stroke, and myocardial infarction) for interventional treatment of carotid artery stenosis with cut-off values of 6% in symptomatic patients and 3% in asymptomatic patients are still valid [8, 9]. Therefore, each new device should be compared in terms of safety against this accepted performance goal in centres with documented high volume and extensive experience in CAS procedures.
Aim
The aim of this study was to evaluate the short- and long-term safety and efficacy of a new generation, self-expanding, open-cell MER™ stent in a group of 100 patients who underwent internal carotid artery stenting.
Material and methods
Setting
Between October 2016 and May 2017, 100 patients underwent carotid artery stenting with different protection devices, with implantation of a new generation, self-expanding nitinol stent (MER™). The study was conducted in four centres performing CAS in Poland. The Ethics Committee at the Beskid Medical Chamber in Bielsko-Biala, Poland approved the study (opinion number: 2015/03/26/2 dated 26.03.2015). The study was registered at ClinicalTrials.gov (NCT03133429).
Qualification and periprocedural medication
All patients provided written, informed consent. Prior to the procedure all patients underwent Doppler ultrasound examination of the carotid arteries. Some patients were scanned with angio-CT of the aortic arch and brain-supplying arteries at the operator’s discretion. Symptomatic patients with ≥ 50% stenosis or asymptomatic patients with ≥ 80% stenosis of ICA were enrolled. All patients were qualified for the procedure by the neurologist and underwent neurological assessment by the same neurologist directly after CAS, at discharge, and at 30 days after the procedure. All patients received dual antiplatelet therapy (aspirin 75 mg/day + clopidogrel 75 mg/day) once daily for at least 3 days before the procedure. Post-procedural antiplatelet therapy included clopidogrel for 3 months and lifelong aspirin.
Device
The MERTM carotid self-expanding stent manufactured by the Balton Company is made of nitinol alloy. The outline of the stent is obtained by means of laser work. A stent is placed at the end of a rapid-exchange 0.014 inch delivery system. After being released from the delivery system, the stent opens up, taking the form of a cylinder. Thanks to its properties, it restores the desirable shape of the lumen of the vessel. The stent is placed between two markers at the distal end of the delivery system. The MER device is pictured in Figure 1.
Procedure
All hypotensive, diuretic, and anti-arrhythmic drugs were withheld on the day of the procedure, and all patients received 500 ml of isotonic saline before and during the procedure. In all patients, CAS was performed with the use of cerebral protection devices.
Self-expanding open-cell nitinol MER™ carotid stents (Balton, Poland) were implanted in all patients.
During the procedure, patients received unfractionated heparin to maintain activated clotting time of 250–300 s. All patients, except those with an implanted pacemaker, received 0.5–1.0 mg of atropine before stent implantation. Fifty-five patients underwent direct stent implantation. Post-dilation was performed in all patients to optimise the angiographic result of the procedure (residual stenosis ≤ 30%). All patients underwent pre- and postprocedural intracranial angiography to exclude periprocedural embolic complications. In most patients (after initial femoral artery angiography) the arterial puncture site was closed using a closure device.
Follow-up
An ultrasound examination was performed before discharge and at 30 days, and 6 and 12 months to check the implanted stent. A neurological consultation was done to assess any neurological incidents that occurred during 30-day and 1-year follow-up.
In addition, data on the occurrence of myocardial infarction and death were collected. The primary endpoint was the 30-day and 1-year major adverse event (MAE) defined as cumulative incidence of death, stroke, and myocardial infarction.
Statistical analysis
Categorical variables are presented as numbers and percentages; continuous variables are expressed as mean ± standard deviation (SD). Differences between groups were compared using Student’s or Welch’s t-test depending on the equality of variances for normally distributed continuous variables. The Mann-Whitney U-test was used for non-normally distributed variables. Normality was assessed by the Shapiro-Wilk test. Equality of variances was assessed using Levene’s test. Categorical variables were compared by Pearson’s χ2 test, or by Fisher’s exact test if 20% of cells had an expected count of less than five. To analyse event-free survival in selected risk groups, Kaplan-Meier curves were drawn. Log-rank statistics were used to test the differences in the outcomes between the groups. Statistical analyses were performed with JMP®, version 14.0.0 (SAS Institute Inc., Cary, NC, USA) and R, version 3.4.1 (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria, 2017. https://www.r-project.org/). A p-value less than 0.05 was considered as statistically significant. This was an observational study, and no formal power calculations were performed to determine the sample size.
Results
Mean age of enrolled patients was 68.5 ±8.2 years, and 44 (44%) patients had a history of neurological incidents during the 6 months preceding CAS (Table I).
Table I
Baseline characteristics of the studied group and comparison between asymptomatic and symptomatic subjects
| Variable | Asymptomatic (n = 56) | Symptomatic (n = 44) | Total (n = 100) | P-value |
|---|---|---|---|---|
| Demographic and clinical characteristics: | ||||
| Male gender | 33 (58.9%) | 28 (63.6%) | 61 (61.0%) | 0.63 |
| Age [years] | 68.40 ±7.83 | 68.32 ±8.7 | 68.36 ±8.20 | 0.73 |
| Hypercholesterolaemia | 43 (76.8%) | 28 (63.6%) | 71 (71.0%) | 0.15 |
| Family history of stroke | 3 (5.4%) | 8 (18.2%) | 11 (11.0%) | 0.06 |
| Current or former smoker | 27 (48.2%) | 30 (68.2%) | 57 (57.0%) | 0.0453* |
| Diabetes mellitus | 19 (33.9%) | 20 (45.5%) | 39 (39.0%) | 0.24 |
| Arterial hypertension | 52 (92.9%) | 39 (88.6%) | 91 (91.0%) | 0.50 |
| SBP [mm Hg] | 153.98 ±24.45 | 150.50 ±21.24 | 152.45 ±23.04 | 0.46 |
| DBP [mm Hg] | 76.77 ±10.46 | 80.43 ±11.88 | 78.38 ±11.20 | 0.23 |
| Previous TIA | 5 (8.9%) | 14 (31.8%) | 19 (19.0%) | 0.0038* |
| Time since previous TIA: | 0.0012* | |||
| < 6 months | 0 (0.0%) | 12 (85.7%) | 12 (63.2%) | |
| 6–12 months | 0 (0.0%) | 1 (7.1%) | 1 (5.2%) | |
| ≥ 12 months | 5 (100.0%) | 1 (7.1%) | 6 (31.6%) | |
| Ipsilateral TIA | 4 (80.0%) | 12 (85.7%) | 16 (84.2%) | 1.00 |
| Amaurosis fugax | 0 (0.0%) | 4 (28.6%) | 4 (21.1%) | 0.53 |
| Previous stroke | 13 (23.2%) | 30 (68.2%) | 43 (43.0%) | < 0.0001* |
| Time since previous stroke: | < 0.0001 | |||
| < 6 months | 0 (0%) | 27 (90.0%) | 27 (62.8%) | |
| 6–12 months | 4 (30.8%) | 0 (0%) | 4 (9.3%) | |
| ≥ 12 months | 9 (69.2%) | 3 (10.0%) | 12 (27.9%) | |
| Stroke type: | – | |||
| Ischaemic | 13 (100.0%) | 30 (100.0%) | 43 (100.0%) | |
| Haemorrhagic | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Ipsilateral stroke | 3 (23.1%) | 26 (86.7%) | 29 (67.4%) | 0.0001* |
| Hemiparesis or hemiplegia | 8 (66.7%) | 24 (80.0%) | 32 (76.2%) | 0.43 |
| Previous MI | 15 (26.8%) | 10 (22.7%) | 25 (25.0%) | 0.64 |
| Previous PCI | 22 (39.3%) | 10 (22.7%) | 32 (32.0%) | 0.07 |
| Previous CABG | 11 (19.6%) | 2 (4.6%) | 13 (13.0%) | 0.0259* |
| Laboratory tests: | ||||
| Hgb [g/dl] | 13.59 ±1.29 | 14.32 ±1.29 | 13.91 ±1.33 | 0.0060* |
| Serum creatinine [mg/dl] | 1.00 ±0.27 | 1.05 ±0.30 | 1.02 ±0.28 | 0.31 |
| eGFR [ml/min/1.73 m²] | 73.48 ±18.80 | 66.66 ±18.30 | 70.75 ±18.79 | 0.07 |
| PLT [× 1000/µl] | 238.46 ±59.84 | 256.89 ±79.94 | 246.57 ±69.64 | 0.36 |
| RBC [× 106/mm³] | 4.52 ±0.43 | 4.72 ±0.47 | 4.61 ±0.46 | 0.0311* |
| TSH [µIU/ml] | 1.89 ±2.08 | 1.44 ±1.05 | 1.74 ±1.80 | 0.63 |
| ALT [IU/l] | 21.59 ±8.65 | 32.85 ±31.97 | 25.63 ±20.76 | 0.33 |
SBP – systolic blood pressure, DBP – diastolic blood pressure, TIA – transient ischemic attack, MI – myocardial infarction, PCI – percutaneous coronary intervention, CABG – coronary artery bypass grafting, Hgb – haemoglobin, eGFR – estimated glomerular filtration rate, PLT – platelets, RBC – red blood count, TSH – thyroid stimulating hormone, ALT – alanine aminotransferase, ASA – aspirin, ACEI – angiotensin convertase enzyme inhibitor.
Mean maximal carotid artery stenoses before and after the procedure were 81.98 ±9.15% and 12.52 ±8.70%, respectively (p < 0.001).
Procedural details are shown in Table II. Proximal protection devices were used in 19 (19%) patients. Procedural success defined as residual carotid artery stenosis ≤ 30% was achieved in 97 (97%) patients. In 2 cases, additional stent implantation was needed. In all patients, stent implantation was followed by stent post-dilation. An additional stent post-dilation with a 0.5 mm larger balloon was performed in 2 (2%) patients due to angiographic criteria of plaque prolapse through the stent struts. Debris in the protection devices was estimated in one centre. Embolic material was found in 15/47 (32%) patients.
Table II
Procedure summary and comparison between asymptomatic and symptomatic subjects
| Variable | Asymptomatic (n = 56) | Symptomatic (n = 44) | Total (n = 100) | P-value |
|---|---|---|---|---|
| Duplex ultrasound | ||||
| %DS (NASCET) | 77.00 ±8.75 | 75.38 ±10.69 | 76.29 ±9.63 | 0.30 |
| Procedural data: | ||||
| Femoral access site | 56 (100.0%) | 44 (100.0%) | 100 (100.0%) | – |
| Target lesion: | 0.06 | |||
| CCA | 5 (8.9%) | 0 (0.0%) | 5 (5.0%) | |
| ICA | 51 (91.1%) | 44 (100.0%) | 95 (95.0%) | |
| Baseline %DS (angiography) | 82.38 ±7.18 | 81.48 ±11.24 | 81.98 ±9.15 | 0.62 |
| RVD [mm] | 5.70 ±0.82 | 5.59 ±1.05 | 5.65 ±0.93 | 0.93 |
| Lesion length [mm] | 18.58 ±8.32 | 15.48 ±7.68 | 17.22 ±8.15 | 0.09 |
| Calcification | 22 (39.3%) | 26 (59.1%) | 48 (48.0%) | 0.0491* |
| EPD used | 56 (100.0%) | 44 (100.0%) | 100 (100.0%) | – |
| EPD device: | 0.13 | |||
| Emboshield | 4 (7.1%) | 1 (2.3%) | ||
| FilterWire | 15 (26.8%) | 16 (36.4%) | ||
| Mo.Ma | 13 (21.2%) | 6 (13.6%) | ||
| Robin | 3 (5.4%) | 7 (15.9%) | ||
| SpiderFX | 15 (26.8%) | 13 (29.6%) | ||
| WIRION | 6 (10.7%) | 1 (2.3%) | ||
| Proximal protection | 13 (23.2%) | 6 (13.6%) | 19 (19.0%) | 0.22 |
| Direct stenting | 26 (46.4%) | 29 (65.9%) | 55 (55.0%) | 0.05 |
| Postdilatation | 56 (100.0%) | 44 (100.0%) | 100 (100.0%) | – |
| %DS post procedure (angiography) | 12.80 ±6.58 | 12.16 ±10.88 | 12.52 ±8.70 | 0.20 |
| Procedural success (%DS ≤ 30%) | 56 (100.0%) | 41 (93.2%) | 97 (97.0%) | 0.08 |
| Periprocedural complications: | ||||
| Death | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Periprocedural stroke | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Periprocedural TIA | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Periprocedural MI | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Dissection | 0 (0.0%) | 1 (2.3%) | 1 (1.0%) | 0.44 |
| Perforation | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Need for 2nd stent implantation | 0 (0.0%) | 2 (4.6%) | 2 (2.0%) | 0.19 |
| Vascular access site complications | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
Representative cases of CAS with the MER™ stent are shown in Figures 2 and 3.
Figure 2
A – Symptomatic 80% right internal carotid artery stenosis. B – Protection with SpiderFX and MER™ stent 6–8 × 30 mm implantation followed by 5.0 × 20 mm balloon postdilatation. C – Excellent angiographic stent apposition with no residual stenosis
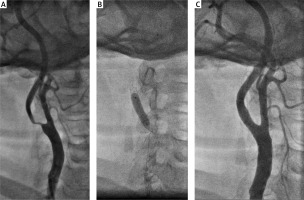
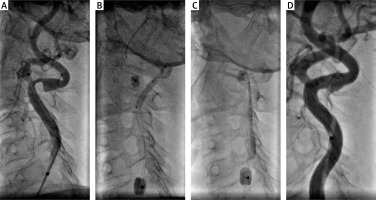
Figure 3
A – Tight symptomatic left internal carotid artery stenosis with severe double angulation > 90°. The use of an 0.014 inch Grandslam support guidewire required due to the extreme angulation of the artery. B – Proximal protection with 8 Fr Mo.Ma – predilatation with 3.0 × 20 mm coronary balloon catheter. C – MER™ stent 7.0 × 20 mm implantation. D – Final optimal angiographic result
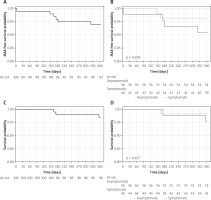
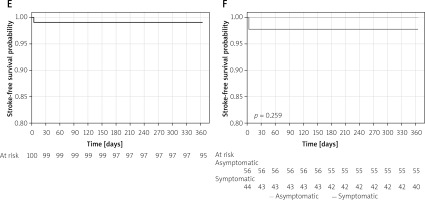
There was a single case of a major stroke at day four post procedure. No periprocedural or in-hospital myocardial infarctions or deaths were observed. There were no cases of death, myocardial infarction, or stroke between discharge and day 30 (Table II, Figure 4). Doppler ultrasound assessment of the implanted stents confirmed optimal stent apposition with maintained patency of all stents at 1 year in surviving patients. During 1-year follow-up 1 patient developed in-stent restenosis that required a repeated procedure. The optimal clinical and angiographic effect was achieved in this case. There were 3 deaths at 1-year follow-up: 1 due to complications after myocardial infarction, 1 due to complications during treatment of acute leg ischaemia, and 1 due to suicide. Finally, Kaplan-Meier survival function showed 97% 1-year overall survival and 99% stroke-free survival. Major adverse event (MAE)-free survival was 94% during 1-year follow-up. No differences between symptomatic and asymptomatic patients were observed (Figure 4), but with 1 event in 100 patients the study was not powered for any statistical evaluation of the potential stent behaviour difference in symptomatic vs. asymptomatic carotid stenosis.
Figure 4
One-year Kaplan-Meier event-free survival curves for patients undergoing carotid artery stenting with MER™ stents. A – MAE-free survival for all patients. B – MAE-free survival for the symptomatic and asymptomatic patients. C – Overall survival. D – Survival for the symptomatic versus asymptomatic patients. E – Freedom from stroke for all patients. F – Freedom from stroke for symptomatic and asymptomatic patients
MAE – major adverse event; cumulative incidence of death, stroke, and myocardial infarction.
Discussion
In a cohort of 100 unselected patients qualified for CAS, we confirmed a good safety and efficacy profile of the implantation of a new MER™ CAS device. More importantly, the achieved results met the established performance goals for percutaneous carotid procedures.
The number of CAS procedures is growing, and new stents, as well as protective devices or access modes (e.g. direct transcarotid access), have proven high efficacy in percutaneous treatment of atherosclerotic changes in the brain-supplying arteries [9–11]. Several years of experience in CAS and the growing significance of proximal devices used for CAS have caused a marked reduction of the periprocedural complication rate and a shift from periprocedural towards postprocedural complications [12].
It is estimated that 2/3 of all complications that occur after the CAS procedure are caused by migration of embolic material through the stent cells. Therefore, adequate stent apposition to the atherosclerotic plaque and artery wall is crucial and can be best obtained with the use of open-cell stents.
A second key element is to continuing dual antiplatelet therapy for three months and acetylsalicylic acid lifelong. The size of the self-expandable MER™ stent cell is approximately 6.2 mm2, so it fits lover value of all open-cell-design stents. This is a great advantage in stent design if one compares the cell area with that of the Acculink (Abbott) stent, at 11.48 mm2. This characteristic may influence the size of embolic material and therefore the clinical consequences caused by migration of atherosclerotic plaque parts to the cerebral circulation.
Bosiers et al. [13] demonstrated that the use of open-cell stents is related to a higher risk of complications in comparison to closed-cell stents. However, our previous studies did not demonstrate differences in immediate and long-term results of CAS between both types of stents if cerebral protection devices and closed-cell stents were used in symptomatic patients and in patients with high-risk lesions [3]. It should be noted that open-cell stents adapt better to tortuous segments of carotid arteries, and therefore they have a well-established position as first-choice stents in patients with tortuous and calcified carotid arteries treated with the endovascular method [14].
In a 2-year observation, Muller-Hulsbeck et al. found no difference in complications between open- and closed-cell stents implanted in the carotid arteries [15]. Recently published results of the largest meta-analysis presented by De Vriest et al. [16] showed no differences in 30-day and long-term complication rates between open- and closed-cell stents.
Today we have a new generation of mesh stents, which can be used safely in high-risk lesions. First reports with Roadsaver and CGuard mesh stents are extremely promising due to their very low 30-day complication rates [6, 7, 17].
In our study using the open-cell MER™ stent, we confirmed a very low complication rate of 1% during 30-day follow-up. It should be noted that one major stroke was diagnosed in a symptomatic patient shortly after an ischaemic incident, with new neurological symptoms at day four after CAS procedure, with partial aphasia that persisted for over 24 h after the incident.
MER™ stents have relatively small area cells, at 6.2 mm2, which could also have influenced the good results of the procedure in the analysed group. During 11 months of follow-up we did not observe any additional strokes. Only 3 deaths were observed, including one due to suicide and another one due to complicated treatment of leg ischaemia.
Nowadays, it is difficult to perform CAS solely with the use of closed-cell stents. The properties of nitinol self-expandable stents are frequently crucial for correct conduction of a CAS procedure. However, the selection of stent used for CAS should be based on the morphology of the atherosclerotic plaque and on the anatomy of the narrowed artery [3, 4, 14]. The MER™ stent could replace the Cristallo Ideale (Medtronic) hybrid stent, which was withdrawn from the market several years ago. Also, the use of the Paladin (ContegoMedical) system with the open-cell carotid stent is an option in high-risk lesions [18].
The great advantage of the MER™ stent is its crossing profile. All sizes of these stents are 5 Fr compatible, which allows the use of a 6 Fr guiding catheter or 5 Fr shuttle sheath. Recently, we have observed a lot of interest in CAS procedures from the radial approach [19]. It is important that this stent can be used from the radial access even for the treatment of very tortuous common or internal carotid arteries.
The 1-year in-stent restenosis rate in our group is lower than the average presented in literature (1% vs. 4.6–6.3%) [20]. The explanation for this finding may be the open-cell design of the stent, which provides adequate stent apposition and routine post-dilatation, giving optimal stent expansion and lumen gain.
A high rate of direct stenting may be a theoretical advantage. However, Lauricella et al. have recently presented data showing that target-diameter predilatation (without further postdiatation) may be associated with a significantly lower rate of macroscopic debris in protection devices [21].
Results from this multi-centre study indicate that MER™ stent-supported carotid angioplasty may be a safe and durable procedure.
Limitations
The main limitation of the study is the fact that it was a one arm, non-blinded, non-randomised study, so a head-to-head comparison of different treatment strategies was not possible.
Moreover, no pre-procedural and post-procedural diffusion-weighted magnetic resonance cerebral imaging [22] was performed, so we could not define the effect of CAS procedures in this study on post-CAS cerebral embolism [23]. Finallly, visualisation in this study was limited to the clinically routine techniques of duplex Doppler and angiography, so we could not evaluate in detail the lumen of the artery post-procedure [24, 25].
Conclusions
Carotid artery stenting with nitinol open-cell MER™ 5F stents is safe. The rate of periprocedural and 30-day complications is lower than the rates accepted by ESC guidelines. The results of 1-year follow-up are favourable and may support the use of this stent in the majority of symptomatic and asymptomatic patients scheduled for CAS.









