Lichen planus (LP) is a chronic inflammatory disease characterized by pruritic, purple, polygonal, and flat-topped papules. It generally occurs in middle-aged adults. The histological finding of LP shows the presence of a dense, thick, band-like lymphocytic infiltrate in the upper dermis along the basement membrane zone. LP affects approximately 1% of the world population with no gender predilection [1]. Although the etiopathogenesis of LP is not yet clear, it involves genetic and environmental factors, as well as the activation of Th1 and IL-23/Th-17 axes [2]. Despite the fact that childhood LP is not common, children do account for estimated 1–4% of LP cases. Moreover, the majority of these reported cases are from India [3]. The oral mucosa is less affected in children with LP [3]. Topical corticosteroids, dapsone, and systemic steroids are the most common treatment options available for childhood LP. However, some patients do not respond well to these treatment options. Here, we report a case of childhood LP responsive to an oral Janus kinase inhibitor (JAKi), upadacitinib.
A 10-year-old boy was presented to our clinic in July 2021 for generalized asymptomatic pigmented plaques forming throughout his body for the past 3 years. He did not have associated systemic and autoimmune pathologies, medications, and dental restorations. His clinical examination revealed multiple pigmented plaques over his body surface, without any oral mucosal and appendageal involvement (Figures 1 A and B). His disease severity score evaluated by Lichen Planus Severity Index (LPSI) was 32 [4]. All routine laboratory tests on blood cell analysis and liver and kidney functions were within their respective normal ranges. His histological findings were basal cell liquefaction and degeneration, as well as lymphocytic infiltrate with melanin incontinence in the upper dermis. Based on his clinical presentations and the histological results (Figure 2), he was diagnosed with childhood LP and treated with topical steroids and oral traditional Chinese medicines for 2 years, including compound glycyrrhizin tablets and total glucosides of white paeony capsules. However, his condition did not show any significant improvement. His quality of life (QoL) was negatively impacted as he became self-conscious owing to the rash. Therefore, we initiated an off-label therapy with upadacitinib (15 mg daily orally) after obtaining informed consent from his parents. The skin lesions of the patient resolved within 2 months and his LPSI score reduced to 20 (Figures 1 C and D). However, his alanine transaminase (ALT) level elevated to 67 KU/l (normal: 9–60 KU/l) and his aspartate aminotransferase (AST) level significantly increased to 191.3 KU/L (normal: 15–40 KU/l). We took him off upadacitinib for ten days and checked back. Both the ALT and AST levels had returned to normal after this break. He was then placed on 15 mg of upadacitinib once every other day. Till the writing of this report, no other adverse events had occurred.
Figure 1
A, B – Multiple pigmented plaques and patches over almost the whole body, C, D – Clinical improvement 2 months after oral upadacitinib
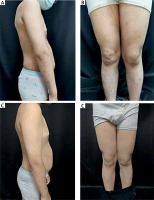
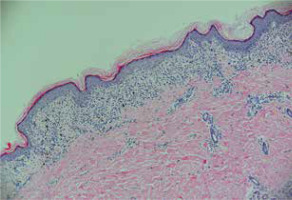
Figure 2
Epidermal basket-weave hyperkeratosis, stratum granulosum hyperkeratosis, liquefaction degeneration of basal cells, and banded infiltration of lymphocytes at the dermo-epidermal junction (HE, 100×)
LP can affect the skin, appendages, as well as mucous membranes. It usually features as pruritus, resulting in functional impairment and difficulty sleeping. In addition to the damaging effects of the disease itself, its high recurrence rate can also severely impact the QoL of patients [5]. In LP, the skin is characterized by T-cell infiltration, apoptosis of epidermal keratinocytes, and a dominant interferon γ (IFN-γ) response. The latter provides a mechanistic basis for the use of JAKi [6]. In contrast, conventional treatments for LP, which include the use of corticosteroids, phototherapy, retinoids, and cyclosporine, have poor efficacy and cause significant side effects. In addition, the clinical course of childhood LP has not yet been well established. Treatment guidelines for the disease also need to be identified. LP in children can differ from that in adults. Moreover, treatment of childhood LP using topical and systemic corticosteroids could lead to significant side effects [3]. Our patient had a history of LP of more than 5 years. He had already undergone topical treatment with corticosteroids as well as systemic therapy with traditional Chinese medicines, which is considered to have fewer side effects. However, none of the two treatments could sufficiently ameliorate his symptoms. Although systemic corticosteroids, either as oral therapy or intravenous pulse therapy, can be considered to control the disease, side effects such as an adverse impact on his height made his parents refuse the treatment. Furthermore, the discontinuation of conventional treatments may lead to the relapse of the condition. JAKi is attracting increasing attention as a promising alternative to conventional treatments for skin diseases, especially those mediated by multiple cytokines such as LP. Some case series and case reports have reported on the safety and efficacy of JAKi in treating LP variants [7]. However, only a few case studies are available on the use of upadacitinib, a highly selective oral JAKi, on patients with LP [8–10]. The present case report shows the high efficacy and potential application of upadacitinib in treating childhood LP.
Upadacitinib is generally well tolerated in adolescents. Hence, it has been approved in China for treating moderate-to-severe skin disorders of adolescents of over 12 years of age. Acne is the most common adverse event associated with the use of upadacitinib. Notably, all acne events are mild or moderate. Although our patient presented with a transient elevation of liver enzymes, it was considered to be rare. Three randomized clinical trials involving 552 adolescents have demonstrated that upadacitinib administered once daily through 16 weeks make the grade 3 liver enzyme (AST) infrequent (the AST was observed in only 1 patient in the 15-mg treatment group and 1 patient in the 30-mg treatment group). Hence, upadacitinib can be considered to be generally well tolerated and safe in adolescents, similar to that observed in adults [11].
In conclusion, we reported a case of childhood LP that demonstrated a consistent and sustained response to oral upadacitinib, in line with the previously published results. Hence, upadacitinib can be considered a treatment option for refractory childhood LP.








