Introduction
Germ cell tumours (GCTs) are the most common testicular neoplasms, and they display highly variable histomorphological patterns. The World Health Organization (WHO) 5th edition 2022 classification of testicular tumours divides testicular GCTs into 2 main groups: those derived from germ cell neoplasia in situ (GCNIS) and those unrelated to GCNIS [1]. The former group is further categorized into the germinoma family of tumours (seminoma), non-seminomatous GCTs (embryonal carcinoma, choriocarcinoma, post-pubertal yolk-sac tumour, and post-pubertal teratoma), and mixed GCTs.
Post-pubertal testicular teratomas are composed of miscellaneous somatic tissues, which originate from one or more of the germinal layers such as endoderm, mesoderm, and ectoderm [2]. The elements produced by teratoma demonstrate variable differentiation ranging from terminally differentiated (e.g. keratinizing squamous epithelium) to immature foetal or embryonic-type tissues. Albeit rarely, somatic-type malignancy may develop in teratoma, especially in metastatic lymph nodes after chemotherapy [3]. The most common histological type arising in the testis is rhabdomyosarcoma, while in the metastatic sites, carcinomas predominate [4]. Other neoplasms, including embryonic type neuroectodermal tumour and nephroblastoma (Wilms tumour), were also reported in association with testicular teratoma, but they are uncommon. The histology of somatic malignancy and the site of its development (primary tumour vs. metastasis) has a significant impact on a patient’s survival [3].
This case report depicts the clinical, immunohistochemical, and histopathological features of testicular teratoma with nephroblastoma.
Case report
A previously healthy 37-year-old patient presented with a left testicular mass. The patient underwent an ultrasonographic examination of the testis, which raised suspicion of testicular malignancy. The levels of tumour markers (serum β-hCG and serum AFP) as well as lactate dehydrogenase were within normal values. Subsequently, the patient underwent left radical orchiectomy, and the postsurgical course was uneventful. X-ray examination of the lungs and abdominal ultrasound were performed without signs of metastases or significant abnormalities.
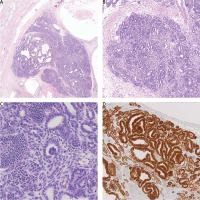
A gross pathological examination of the testis revealed a solid-cystic tumour measuring up to 2.5 cm. The tumour was well demarcated, and no invasion of the testicular hilum, spermatic cord, or epididymis was noted. Microscopically, the tumour was composed of numerous cystic elements lined by stratified squamous epithelium and glandular intestinal-type epithelium, small foci of immature cartilage, and immature teratomatous stroma. Moreover, several foci of abundant epithelial lesion-forming tubules, abortive structures resembling foetal glomeruli, and islands of primitive cells embedded in the fibrous stroma were noted (Fig. 1). The largest diameter of one of these foci was 6 mm. The mitotic index was 35/10 HPF. Immunophenotype of this component was as follows WT-1 (nuclear+), PAX-8 (+), CK AE1/AE3 (+), focal synaptophysin (+), chromogranin (–), CD30 (–), PLAP (–), p53 (–), OCT-4 (–), AFP (–), and desmin (–). No WT-1 or PAX-8 expression was noted in teratomatous elements. There was no lymphovascular invasion. Germ cell neoplasia in situ was present in the seminiferous tubules outside the tumour. The surgical margin of the spermatic cord was free of cancer. The final histopathological diagnosis based on typical morphology and immunophenotype was teratoma with nephroblastoma (somatic-type malignancy). The pathological stage was defined as pT1 according to Classification of Malignant Tumours, 8th Edition.
Fig. 1
Nephroblastoma component of testicular teratoma on low (A), intermediate (B), and high (C) magnification. Strong nuclear WT-1reaction in nephroblastoma cells (D)
The patient opted for surveillance and did not receive any further treatment. Two years after the initial diagnosis, he is without any complaints or signs of recurrence.
Discussion
Nephroblastoma is the most frequent renal malignancy in children, and its occurrence in an adult is extraordinary [5]. Microscopically, it classically displays a triphasic pattern with epithelial elements recapitulating the foetal kidney, blastema, and stroma. Tumour cells typically express nuclear WT-1 and PAX-8. Nephroblastoma is derived from nephrogenic rests that are abnormally persistent foci of embryonal cells originating from intermediate mesoderm present within developmentally normal kidneys. They are seen in approximately 1% of term infant autopsies, and in 25–40% of kidneys with nephroblastoma [6]. Extrarenal nephroblastoma is a rarity and poses a significant diagnostic challenge for a pathologist, especially in pure blastemal type mimicking other small round blue cell tumours. Most cases occur in children and probably originate from ectopic nephrogenic rests or nephrogenic blastemal cells scattered in the craniocaudal migration pathway of primitive metanephros cells [7]. Reported sites include the retroperitoneum, inguinal area, pelvis, genitourinary tract, and mediastinum [7]. Association of extrarenal nephroblastoma with a substantial teratoid component (so-called teratoid Wilms tumour) is even less common and possibly represents the extremal heterologous element differentiation, not a separate entity [8]. On the other hand, some nephroblastomas may develop within well-defined teratoma, and such cases could be designated as teratoma with nephroblastoma [9]. Nevertheless, clear histopathological criteria distinguishing teratoid nephroblastoma from teratoma with nephroblastoma do not exist, and the use of these terms in the literature is inconsistent.
We reported a case of an obvious nephroblastic component within a classic testicular post-pubertal teratoma coexisting with GCNIS, supporting its origin from primordial germ cells. According to the current WHO classification, GCT with somatic malignancy is defined as an expansile or infiltrative growth of the epithelial or mesenchymal component measuring ≥ 5 mm [10]. In our case the largest focus of nephroblastoma measured 6 mm, meeting these criteria. Only a few similar cases were reported in the literature. We found 7 cases of nephroblastoma arising in association with primary testicular teratoma, one case of pure testicular nephroblastoma with evidence of germ cell origin, and 14 cases developing in metastatic sites of testicular GCTs [3, 11–19] (Table 1). Among patients with nephroblastoma developing in primary testicular GCTs, 2 patients died of the disease, one was alive with disease, and 3 had no evidence of disease in follow-up. In one case no information on follow-up was available. All patients with favourable outcomes had stage I at diagnosis and received no further treatment, similarly to our case. This suggests that orchiectomy and surveillance is an adequate approach in stage I testicular GCTs with nephroblastoma. It is unknown if, in advanced stages, treatment protocols for paediatric Wilms tumours could be an option for testicular GCTs with nephroblastoma. In addition to teratoma and nephroblastoma, 4 cases exhibited other components, namely rhabdomyosarcoma, yolk sac tumour, and embryonal carcinoma. Gillis et al. reported a unique case of pure testicular nephroblastoma associated with GCNIS, the presence of isochromosome 12p, and biallelic expression of imprinted genes, proving its origin from primordial germ cells [11].
Table 1
Cases of nephroblastoma associated with testicular germ cell tumours
| Number | Author | year | Age | Location | Histopathology | Treatment | Follow-up (months)* | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Gills et al. [11] | 1994 | 30 | Testis | WT* + GCNIS | Surgery, surveillance | 25 | NED |
| 2 | Emerson et al. [12] | 2004 | 22 | Testis | T + RMS + WT | Surgery, CTx | 21 | NED |
| 3 | Vanasupa et al. [18] | 2007 | 18 | Testis | T + YST + EC + RMS + WT | Surgery, surveillance | 4 | NED |
| 4 | Colecchia et al. [19] | 2011 | 39 | Testis | T + RMS + WT | Surgery, CTx | 20 | DOD |
| 5 | Keskin et al. [14] | 2011 | 19 | Testis | T + WT | Surgery, CTx | 18 | DOD |
| 6 | Alatassi et al. [13] | 2016 | 19 | Testis | T + YST + EC + WT | Surgery, CTx | 6 | AWD |
| 7 | Kromka et al. [15] | 2018 | 50 | Testis | T + WT | Surgery, surveillance | 15 | NED |
| 9 | Current** | 2023 | 37 | Testis | T + WT | Surgery, surveillance | 24 | NED |
| 9 | Carney et al. [16] | 1975 | 41 | LN | T + RCC + WT | Surgery, RT | 54/2 | DWD |
| 10 | Ulbright et al. [17] | 1984 | 19 | LN | T + WT | Surgery, CTx | ?/10 | NED |
| 12 | Ulbright et al. [17] | 1984 | 19 | LN, OM | T + WT | Surgery, CTx | ?/36 | AWD |
| 12 | Michael et al. [20] | 1998 | 23 | LN, OM | T + WT | Surgery, CTx | 72/36 | AWD |
| 13 | Michael et al. [20] | 1998 | 20 | LN, OM | T + WT | Surgery, CTx | 60/60 | NED |
| 14 | Michael et al. [20] | 1998 | 26 | LN | T + PNET + WT | Surgery, CTx | 10/5 | DOD |
| 15 | Michael et al. [20] | 1998 | 29 | LN | T + WT | Surgery, CTx | 68/68 | NED |
| 16 | Michael et al. [20] | 1998 | 25 | LN | T + YST + WT | Surgery, CTx | 108/108 | NED |
| 17 | Michael et al. [20] | 1998 | 20 | LN | T + WT | Surgery, CTx | 120/84 | NED |
| 18 | Michael et al. [20] | 1998 | 20 | LN | T + WT | Surgery, CTx | 108/108 | NED |
| 19 | Michael et al. [20] | 1998 | 29 | OM | T + WT | Surgery, CTx | 144/144 | NED |
| 20 | Michael et al. [20] | 1998 | 19 | OM | T + WT | Surgery, CTx | 36/30 | DWD |
| 21–24 | Hwang et al. [3] | 2023 | n/a | 3 cases of WT arising in association with testicular GCT, 1 case in the primary tumour; 2 cases in the metastatic site, other information n/a | ||||
AWD – alive with disease, CTx – chemotherapy, DOD – died of disease, DWD – died with disease, EC – embryonal carcinoma, GCNIS – germ-cell neoplasia in situ, LN – lymph node metastasis, n/a – not available, NED – no evidence of disease, OM – organ metastasis, PNET – primitive neuroectodermal tumour, RMS – rhabdomyosarcoma, RT – radiation therapy, T – teratoma, WT – nephroblastoma, YST – yolk sac tumour
The majority of nephroblastomas that arise in metastatic sites of testicular GCT are typically combined only with teratoma, except for 3 cases where components of primitive neuroectodermal tumour, yolk sac tumour, and renal cell carcinoma were present. Michael et al. suggested that chemotherapy may be a factor triggering the development of the nephroblastoma component in metastatic GCTs – all but one patient in their cohort received adjuvant chemotherapy after orchiectomy [20]. This phenomenon could be explained by the selective elimination of chemosensitive GCT with the subsequent outgrowth of chemoresistant somatic tissue and different microenvironment of metastases [10]. In patients with available outcomes (n = 11), 3 patients died, 2 were alive with disease, and 6 had no evidence of disease. Despite the limited evidence, the outcomes of nephroblastoma in the setting of metastatic GCT appear to be relatively good when compared to embryonic type neuroectodermal tumour (formerly PNET) in the same clinical scenario [20].
Differential diagnoses of testicular nephroblastoma include other somatic-type malignancies, especially neuroectodermal tumour and rhabdomyosarcoma, embryonal carcinoma, yolk sac tumour, and sex cord-stromal tumour. Our case and the literature data demonstrate that suspicion of nephroblastoma in GCT can be readily made based on morphology in routine haematoxylin-eosin staining, but strong nuclear positivity for WT-1 and PAX-8 supports the diagnosis. Of note, nephroblastoma was also reported in association with ovarian teratomas [21].
Conclusions
In conclusion, this case report illustrates the rare occurrence of teratoma with nephroblastoma, a challenging diagnosis due to its unusual presentation. The identification of characteristic morphology recapitulating foetal kidney and the nuclear expression of WT-1 and PAX-8 supported the diagnosis of nephroblastoma in this case. The lack of lymphovascular invasion and normal tumour markers are favourable prognostic factors for this patient, who chose surveillance as the follow-up option. This case also emphasizes the value of careful histopathological examination and immunohistochemical analysis for accurate diagnosis, which can guide optimal treatment and surveillance strategies for patients.








