Introduction
During the past two and a half years, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread around the world, affecting the immunologically naïve population, and causing notable morbidity and mortality [1]. SARS-CoV-2 is classed in the subfamily Coronavirinae in the family of Coronaviridae. The trans-membrane spike (S) glycoprotein that makes up the spikes on the viral surface is responsible for penetration of the virus into the host cells. The S1 and S2 subunits represent a part of the S-glycoprotein, where S1 contains the receptor-binding domain (RBD) that binds to the cell’s receptor, while S2 contains the fusion peptide. S-glycoprotein is the most important focus of neutralising antibodies upon infection and a target of treatment and vaccine design [2].
SARS-CoV-2 infection or vaccination against COVID-19 provides the immune response and protection against infection or reinfection and reduces the risk of clinically significant outcomes. According to Lumley et al. [3], seropositive individuals have 89% protection from reinfection, while the estimated efficacies of different types of vaccines are 50% to 92% [4]. However, the period of immunity conferred by vaccination is still unknown. New virus variants that emerge may escape vaccines or convalescent immune responses [5]. The vaccines are important for raising the immunity, avoiding severe disorders caused by SARS-CoV-2, reducing the burden on healthcare systems, and decreasing the economic cost [6]. Vaccines developed by Pfizer-BioNTech (BNT162b2/Comirnaty) and Moderna (mRNA-1273/Spikevax) with > 90% efficacy against symptomatic infection, and J&J/Janssen (Ad26.COV2. S) with efficacy of 60-70% against symptomatic infection, have been approved in the United States by the Food and Drug Administration (FDA), while the European Medicines Agency (EMA) has additionally permitted a fourth vaccine from Oxford/AstraZeneca (AZD1222/Vaxzevria) (efficacy of 60-70% against symptomatic infection) [7]. Sinopharm COVID-19 vaccine or BBIBP-CorV, as an inactivated vaccine, was the first Chinese vaccine authorised by the World Health Organisation (WHO) for emergency use with efficacy of 79% [8]. Sputnik V vaccine is a recombinant vaccine developed by Gamaleya Research Institute, Russia, and its effectiveness against COVID-19 has been shown to be 91.6% [8].
The real-world praxis with SARS-CoV-2 vaccination at health facilities has shown a marked decrease in the incidence of infections among medical staff members [9]. Because of their engagement in response to the pandemic and of the higher risk of being infected due to occupational exposure, healthcare employees are considered as a key target group for COVID-19 vaccination in most countries, including the Republic of Srpska. Our recently published study shows that the medical personnel in primary healthcare had a high titre of SARS-CoV-2 antibodies after receiving one or two doses of vaccines [10].
The nature and durability of the protective immunity is important in the context of risk evaluation for reinfection and vaccine development. The vaccines are reported to show a high level of effectiveness in preventing the symptoms and spread of the SARS-CoV-2 infection, even after the first dose [11]. The SARS-CoV-2 infection, as well as the vaccines, induce the development of specific antibodies, but their sustainability is still an object of investigation [12].
In contrast to other countries, we have been able to obtain five different types of vaccines for our population. This gave us the opportunity to compare the immune response to different vaccines. The aim of this study was to examine the titres of anti-spike immunoglobulin (IgG) antibody among medical personnel who were fully vaccinated with one of the five COVID-19 vaccines (BNT162b2-Pfizer- BioNTech vaccine, BBIBP-CorV-Sinopharm vaccine, CoronaVac-Sinovac vaccine, Gam-COVID-Vac-Sputnik V vaccine, and Oxford/AstraZeneca vaccine), and to compare them with antibody titres of COVID-19 convalescents.
Material and methods
Study design and participants
This study cohort included healthy medical personnel from the University Clinical Centre (UCC) of the Republic of Srpska, Banja Luka, the Republic of Srpska, Bosnia and Herzegovina who were vaccinated with at least two doses. Participation in the study was offered to all UCC employees including physicians, nurses, laboratory technicians and administration staff. A total of 261 participants were enrolled in this study between February and September 2021. The anti-SARS-CoV-2 IgG antibody responses following immunisation with one of the five different types of COVID-19 vaccines were compared, as well as with antibody titres of participants with previous natural COVID-19 infection. Five groups of participants who were vaccinated (37 individuals vaccinated with BNT162b2, 17 with BBIBP-CorV, 154 with Gam-COVID-Vac, 11 with Sinovac and 8 with Oxford/AstraZeneca) were matched by sex and age categories.
Patients who suffered COVID-19 infection had mild clinical symptoms such as: fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhoea, loss of taste and smell, but with no clinical signs of pneumonia. Among the vaccinated participants, the anti-SARS-CoV-2 IgG antibody titres were measured 21 days after the first dose and 60 and 180 days after the second dose of the vaccine. All COVID-19 unvaccinated participants enrolled in the study had a prior real-time polymerase chain reaction (RT-PCR)-confirmed SARS-CoV-2 infection. The antibody titres measured 60 days after the second dose of the vaccine, i.e., 90 days following the first dose, were compared with the antibody titres which were measured 90 days after the confirmed COVID-19 infection of the recovered patients. These two groups were comparable regarding the dynamics of antibody production. Immunocompromised patients and patients with chronic diseases were excluded from the study, due to the possible influence on clinical outcomes. The immunised participants were vaccinated to induce synthesis of neutralising antibodies against the viral S glycoprotein. Also, our participants were vaccinated with inactivated vaccines (BBIBP-CorV-Sinopharm vaccine, CoronaVac-Sinovac vaccine) that induce the production of antibodies to other relevant virus antigens.
All study participants completed the questionnaire designed for this study related to their demographic and epidemiological characteristics. Informed consent was obtained from all subjects involved in the study. The laboratory technicians were responsible for collecting the 5 ml samples of venous blood for serological testing. After coagulation, the samples were centrifuged at 3,000 rpm for 5 minutes and the sera were transported to the Centre for Biomedical Research, Faculty of Medicine, University of Banja Luka and stored at –20°C until further serological analysis. The study was done in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Medicine Foča, University of East Sarajevo (Decision number: 01-2-8, dated 6 November 2020).
Detection of SARS-CoV-2 antibodies
Anti-SARS-CoV-2 IgG antibodies were identified using the automated enzyme-linked immunosorbent assay (ELISA) method with a Euroimmun ELISA Analyser I-2P (EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany). Serum samples were analysed with a commercial anti-SARS-CoV-2 ELISA (IgG) kit (code EI 2606-9601 G) according to the producer’s instructions. The microplate wells were covered with an S1 domain of the spike protein of SARS-CoV-2. The results were assessed as a ratio of the extinction of the sample over the extinction of the calibrator. According to the manufacturer’s performance characteristics, the test sensitivity and specificity were 90% and 100%, respectively.
Statistical analysis
Descriptive statistics were used to analyse and describe participants’ characteristics. Categorical variables are presented as absolute frequencies and percentages (%) and continuous data as median with interquartile range (IQR). For the analysis of numerical data, the skewness and kurtosis values as well as a visual inspection of the histogram were used. In the event of deviations from the normal data distribution, the Kruskal-Wallis test was used. The Friedman rank-sum test was used for analysing the seroconversion rate for each group of vaccinated individuals. The Pearson χ2 test was used to compare the frequency of occurrence of the analysed categorical variables (male, female, age group) when observing one or more independent samples. Statistical hypotheses were tested at the significance level (α) of p < 0.05 and p < 0.001. Statistical analysis was done using EZR for Windows XP (Version 2.3-0).
Results
There were 261 participants in this study and 227 (86.9%) of them received at least two doses of any of the five types of COVID-19 vaccines, while the remaining 34 participants were those who recovered from COVID-19 and did not receive any vaccine at that time. However, the age-related information for two unvaccinated patients was missing, and the total number of patients defined by age in this group was 32. Of the 227 vaccinated participants, 37 (16.3%) were vaccinated with the Pfizer-BioNTech vaccine, 17 (7.5%) with Sinopharm, 154 (67.8%) with Sputnik V, 11 (4.9%) with Sinovac and 8 (3.5%) with the Oxford/AstraZeneca vaccine. There were no statistically significant differences in gender ratios between the vaccinated and unvaccinated participants, but differences were noted in distribution of age groups (Table 1).
Table 1
Demographic features of studied participants and COVID-19 seroprevalence
| Characteristic | Participants | P-value | ||
|---|---|---|---|---|
| Vaccinated | Unvaccinated | |||
| Gender, n (%) | ||||
| Male | 56 (24.7) | 13 (58.3) | 0.1 | |
| Female | 171 (75.3) | 21 (36.1) | ||
| Age, n (%) | ||||
| 20-40 | 91 (40.1) | 6 (17.7) | 0.02* | |
| 41-60 | 114 (50.2) | 19 (55.9) | ||
| ≥ 61 | 22 (9.7) | 7 (20.6) | ||
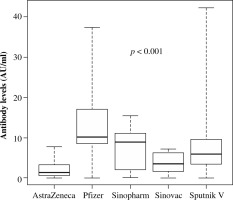
Evaluation of antibody titres among five vaccinated groups showed statistically significant differences in antibody titres 21 days after the first dose. The median antibody titre of 10.0 AU/ml was found in the group vaccinated with the Pfizer-BioNTech vaccine, while in participants vaccinated with Sputnik V, Sinopharm, Sinovac and Oxford/AstraZeneca vaccines the median antibody titres were 9, 7.2, 6.8 and 1.4 AU/ml, respectively (p = 0.01). Sixty days after the second dose of the vaccine, the median antibody titres in participants vaccinated with Pfizer-Bio- NTech, Sinopharm, Sputnik V, Sinovac and Oxford/Astra- Zeneca vaccines were 12, 9.3, 5.9, 4.6 and 2.5 AU/ml, respectively (p < 0.001) (Fig. 1).
Fig. 1
Antibody immunoglobulin G levels of different vaccines measured 60 days after the second dose. The box plot of the median antibody titres after 60 days of the second dose of the vaccines showed statistical significance between them (p < 0.001)
In addition, the comparison of antibody levels among the vaccinated participants 180 days after the second dose of vaccines showed that the Pfizer-BioNTech vaccine induced the highest antibody titre (median = 9.0), followed by those vaccinated with Oxford/AstraZeneca (median = 6.4), Sinopharm (median = 5.6), and Sputnik V (median = 4.7), respectively (p = 0.01).
To analyse the changes in antibody titres at different time-points for each group of vaccinated individuals the Friedman test was used. A statistically significant difference was found between the antibody levels in patients vaccinated with Sputnik V at defined points of time (p < 0.001). Post hoc testing showed that 21 days after the second dose of vaccine, the median antibody titre in participants vaccinated with Sputnik V was significantly higher than the median antibodies titre after 60 days and 180 days after the second dose of the Sputnik V vaccine (p = 0.02, p = 0.0001, respectively). No such differences were found in the groups vaccinated with Pfizer-BioNTech (p > 0.05), Sinopharm (p > 0.05) or Sinovac (p > 0.05) (Table 2).
Table 2
Immunoglobulin G antibody levels of four different vaccines measured at defined points of time after vaccination
| Post vaccination time (days) | Pfizer-BioNTech | Sputnik V | Sinopharm | Sinovac | ||||
|---|---|---|---|---|---|---|---|---|
| Median (AU/ml) | p | Median (AU/ml) | p | Median (AU/ml) | p | Median (AU/ml) | p | |
| 21 | 10.0 | 0.6 | 9.0 | < 0.001* | 7.2 | 0.6 | 6.8 | 0.07 |
| 60 | 12.0 | 5.9 | 9.3 | 4.6 | ||||
| 180 | 9.0 | 4.7 | 5.6 | 0 | ||||
Significant differences in antibody levels 60 days after the second dose of vaccines were not observed between gender and age groups in the same Pfizer-BioNTech and Sputnik V vaccine populations, but a significant difference was noted in the Sinopharm vaccine group (Table 3). Due to the small number of participants vaccinated with Sinovac and Oxford/AstraZeneca, these data were not included in further analyses.
Table 3
Immunoglobulin G antibody levels of three most used vaccines 60 days after administration of the second dose stratified by gender and age
In order to analyse the antibody titre levels between the different vaccine types, the participants were clustered in the group that received the mRNA vaccine (Pfizer- BioNTech), the group that received one of the vector vaccines (Sputnik V or Oxford/AstraZeneca), and the group that received one of the inactivated vaccines (Sinovac or Sinopharm vaccines). Detailed data on antibody titres for each of these three vaccine types are presented in Table 4. The data showed significantly higher antibody titres in individuals vaccinated with the mRNA vaccine compared to those vaccinated with inactivated and vector vaccines (p < 0.05). However, when the participants were stratified by gender and age, there were no differences in antibody levels related to the vaccine type (Table 4).
Table 4
Immunoglobulin G antibody levels 60 days after the second dose according to vaccine type stratified by gender and age
Regarding the vaccine type, it was noted that 88.5% of individuals were seropositive after the mRNA vaccine, 86.2% were seropositive after vector vaccines and 71.4% were seropositive after inactivated vaccines 60 days after the second dose. There were no differences in the efficacy between these three vaccine types (p = 0.1).
Out of the total number of participants, only 109 of them (48%) received the third dose of the vaccine. The COVID-19 infection was confirmed in just 7 participants in this group (6.4%). Out of a total of 227 individuals who received the second dose of the vaccine, in 26 of them (11.5%) the COVID-19 infection after complete vaccination was confirmed. There was no difference between the participants who received the third dose of the vaccine and the participants who received the second dose of the vaccine in relation to the occurrence of COVID-19 infection (p = 0.2).
The analysis showed that the median antibody titre of vaccinated participants 60 days after the second dose of vaccine, which is 90 days after the first dose, was 7.06 AU/ml, and the median antibody level of convalescents 90 days after the positive RT-PCR was 5.76 AU/ml. There was no statistically significant difference between these two groups (p = 0.8).
Discussion
In Western European countries the percentage of the vaccinated adult population is higher than that in Eastern European countries [13]. However, the majority of Western countries were vaccinated either with the mRNA type (Pfizer/Moderna) or with the vector type (AstraZeneca) of vaccine. To the best of our knowledge, this study is the first to analyse the immune response related to five different types of COVID-19 vaccines that were available in the Republic of Srpska in comparison to the immune response of the natural SARS-CoV-2 infection. Immunoassays mostly measure antibodies to the S-glycoprotein or its fragment such as the receptor binding domain (RBD). The ELISA method determines the total sum of antibodies that attach to virus proteins including neutralising antibodies. Tests for detection of neutralising antibodies are also in use, but these tests are not standardised. The neutralising antibody levels as calculated by neutralisation tests are related to antibody titres that are determined by “binding” assays [14]. In the present study the ELISA method, which calculates the quantity of anti-SARS-CoV-2 spike IgG, was used.
In the case of COVID-19 infection, it was shown that only antibodies targeted at the S protein can neutralise the virus and avoid further infection [15]. The SARS-CoV-2 vaccines include at least part of the S-glycoprotein such as the S1 domain or the RBD. It has been shown that the immune response against vaccinal S-glycoprotein significantly differs according to the vaccine type, showing higher seroconversion in mRNA-vaccinated individuals in comparison to other types of vaccines [16].
The results of our study showed the highest antibody levels measured in the group vaccinated with Pfizer-BioNTech. Petrović et al. noted maximum titres in participants vaccinated with Pfizer-BioNTech, followed by Sputnik V and Sinopharm [14]. In the present study, the highest anti- body titres were found in those vaccinated with Pfizer- BioNTech, followed by Sputnik V and Sinopharm 21 days after the first dose of the vaccine, and 60 days after the second dose of the vaccine according to the antibody levels between the groups vaccinated with different vaccine platforms. After 180 days of the second dose of the vaccine the antibody titre was highest in the participants vaccinated with Pfizer-BioNTech, followed by Oxford/AstraZeneca and Sinopharm, and Sputnik V. Comparable differences in antibody titres related to different vaccine types have been reported [14].
The reported divergences in immunogenicity can be explained by the different platforms used to make vaccines. Pfizer-BioNTech vaccine includes mRNA encoding the S-glycoprotein, which is highly immunogenic, wherein the mRNA is stored in a lipid nanoparticle that ensures that the mRNA penetrates more easily into the cytoplasm of the host cells without being degraded by enzymes present in tissues. Adjobimey et al. [16] reported that the vectored vaccines such as Sputnik V and Oxford/AstraZeneca provoked a comparable titre of anti-SARS-CoV-2 specific IgG antibodies. Our results showed weaker efficacy of Oxford/AstraZeneca compared to Sputnik V after the first dose of these vaccines. However, 180 days after the second dose of the vaccine, participants who received Oxford/AstraZeneca or Sinopharm vaccines had higher anti- body titres compared to the individuals vaccinated with Sputnik V. The Sinopharm vaccine may cause production of antibodies to proteins of SARS-CoV-2 other than those against S-glycoprotein. It could be of significance for protection against variants with mutations in the spike region. Hence, induction of the immune response by vaccines depends on host factors and vaccine components. The selection of the vaccine type defines the immunogenic effectiveness of the viral proteins that represent the constituent part of the vaccine. Also, the selection of the appropriate vaccine platform determines that an immune adjuvant is required [17].
When comparing antibody levels measured 21 days after the first dose of vaccine and 60 days after the second dose of vaccine, antibody levels following Sputnik V decreased at a faster rate than the other vaccines. It was also noted that the antibody levels in the group vaccinated with Oxford/Astra Zeneca was higher 180 days after the second dose of the vaccine than 60 days after the second dose of the vaccine. According to the results, Sinopharm and Oxford/Astra Zeneca vaccines are more effective than Sputnik V. This may be due to variations in the platform of these vaccines. Since the COVID-19 infection was new to humanity, it was not clear which vaccine strategies would be the most successful [18]. Studies on vaccinated participants at later time-points are necessary to identify the vaccine that produces the longest-lasting antibody titre.
The Pfizer-BioNTech, Sputnik V, and Sinopharm vaccines induced higher levels of antibody titres in the older population and in females but not at significant levels in groups vaccinated with Pfizer-BioNTech and Sputnik V. Negative correlations between age and antibody expression in Sputnik V, and Sinopharm vaccinated individuals were found by Adjobimey and co-workers [16]. Immunosenescence is a process of immune dysfunction that happens with age and includes changes in the lymphatic organs, leading to a decreased number of naïve T cells, as well as the production of antibodies, which is related to the development of infections, autoimmune diseases, and malignant tumours, as well as lower efficacy of vaccines [19]. The higher level of antibodies in older vaccinated participants in our study seems to refute this statement. The reason for this result may be the sample size of participants in the age group ≥ 61. Besides, Petrović et al. [20] stated that Pfizer-BioNTech, Sputnik V, Sinopharm, and Oxford/Astra Zeneca vaccines had high effectiveness in preventing SARS-CoV-2 infection in the elderly shortly after their administration.
The anti-S antibody levels in participants of this study increased more quickly after vaccination with Pfizer than after Oxford/Astra Zeneca. This was also shown in the study of Iacobucci [21]. In the present study, participants vaccinated with Sputnik V also experienced a decrease in antibody levels, but they were also seropositive 180 days after the second dose of vaccine (6 months from the first dose of the vaccine). In participants vaccinated with Pfizer-BioNTech antibody levels decreased over a period of 6 months, but seropositivity remained. A longitudinal study from Argentina showed that the 118 volunteers vaccinated with two doses of the Sputnik V vaccine had a significant decrease in IgG antibody levels over a period of 6 months, but all subjects remained seropositive [22].
Sinopharm and Sinovac vaccines have been the most widely used vaccines, being registered in 88 and 53 countries, respectively [23]. According to literature data, efficacies of inactivated vaccines Sinopharm and Sinovac increases with the number of vaccine doses [24]. This is contradictory to the results of the present study, since no significant difference was observed in the increase in anti- body levels after the administration of the second dose of the vaccine. Non-viral vaccine platforms require multiple vaccinations to trigger protective immunity, while one dose of live virus vaccines has the capability to provoke ‘one-shot’ immunity. Inactivated virus vaccines must be sometimes inoculated with suitable adjuvants and by repetitive vaccination in order to reach full usefulness [17].
In the present study, participants who were vaccinated with the mRNA vaccine had significantly higher antibody levels compared to participants vaccinated with inactivated and vector vaccines. Polack et al. reported that the effectiveness of the mRNA vaccine was 95% [25]. Also, the data of Adjobimey et al. [16] showed that the mRNA vaccinated groups exhibited the highest neutralisation potential among all the groups. Voysey et al. [26] reported that the vector vaccine achieved an effectiveness rate of 70.4%. Also, we analysed the seroconversion rates in vaccinated persons with three types of vaccine (mRNA, vector, inactivated vaccines). The percentage of participants vaccinated with inactivated vaccines was 71.4%, with vector vaccines 86.2%, and with the mRNA vaccine 88.5%. For the mRNA vaccine, our results are similar to the results obtained in the initial trial [27]. According to the data from the literature the effectiveness of inactivated vaccines ranges between 72% and 78% [28].
Almost half of the participants in the present study received the third dose of the vaccine. The analysis showed that there was no difference between the participants who received the second and the participants who received the third dose of the vaccine in relation to COVID-19 infection. Immune protection against COVID-19 infection depends on the quality, quantity, and duration of antibody levels throughout the disease.
It was noted that the antibody titres in this study were higher in vaccinated groups compared to the convalescents, but without statistical significance, which can be explained by the fact that the patients included in the present study had mild clinical symptoms of COVID-19 infection. This is consistent with other studies that demonstrated higher antibody titres in vaccinated groups compared to convalescents [29]. Israel et al. observed higher titres in the first month following the second vaccination dose than in convalescent patients after SARS-CoV-2 infection [30]. They also found that BNT162b2 vaccine provoked much higher antibody titres three months after vaccination compared to the titres from convalescents, but with a faster fall of antibody titres in vaccinated patients compared to those infected with the SARS-CoV-2 virus [30]. Studies of L’Huillier et al. [31], and Cho et al. [32] also showed that the antibody titre was higher after vaccination compared to the antibody titre after a previous infection.
It is known that IgM and IgG are not predictors of disease severity, but the peak antibody titres are positively associated with the COVID-19 disease severity [33]. Choteau et al. [34] found in their research that a higher titre of virus-specific antibodies was associated with severe COVID-19 infection compared to asymptomatic patients. They also found that severe infection is not associated with a defective immune response, and that in recovered patients antibodies were still detected more than three months after the COVID-19 infection. According to reports in the literature, antibodies in recovered patients persist for 3 to 6 months after infection, and in most patients, they can be detected even 8 to 9 months after infection [35]. It was observed that vaccination of convalescents led to 2-10-fold higher neutralisation titres than those present in naïve individuals after primary vaccination. Also, vaccination of convalescents resulted in a higher neutralisation titre than that present in early convalescents [36]. The present study showed that the COVID-19 convalescents maintained detectable anti-SARS-CoV-2 IgG antibodies 90 days after the beginning of infection. It is confirmed that vaccination of previously recovered patients will provide more permanent protection against variants of SARS-CoV-2 [36]. A question which was not answered is the time interval of these immune responses. The results of some authors showed that there is no significant difference in the decline of antibody levels in the first months between vaccinated persons and those who have recovered from COVID-19 infection [37]. However, this issue has to be evaluated in further studies.
A limitation of this study is the small and different sample sizes between the groups of vaccinated and unvaccinated participants. This might be the reason that some results obtained in this study are not fully comparable with other studies.
Conclusions
The present study compared the efficacy of the five types of anti-SARS-CoV-2 vaccines currently available in the Republic of Srpska, Bosnia and Herzegovina: BNT162b2-Pfizer-BioNTech vaccine, BBIBP-CorV-Sino- pharm vaccine, CoronaVac-Sinovac vaccine, Gam-COVID-Vac-Sputnik V vaccine, and Oxford/AstraZeneca vaccine. Our findings indicated that the Pfizer-BioNTech vaccine caused the highest titres of specific IgG antibodies, followed by Sputnik V and Sinopharm. The results also confirmed that vaccination with all five types of vaccines provided a significant immune response in the majority of vaccinated participants. High rates of seropositivity were detected after vaccination with the mRNA and vector vaccines, while the percentage of seropositive participants vaccinated with inactivated vaccines was lower. The results of this study showed that the antibody levels were higher in vaccinated groups compared to the convalescents but without statistical significance.


