Introduction
Kidney cancer (also known as renal cell carcinoma) currently accounts for approximately 2–3% of malignancies. The peak incidence occurs between the ages of 60 and 70 years, and men are affected approximately 1.5 times more often than women [1]. Approximately 270,000 new cases are diagnosed each year worldwide, and approximately 116,000 patients die annually from this type of cancer [2]. The incidence of kidney cancer is highest in highly developed countries, which is probably related to better access to healthcare and, in particular, better access to diagnostic tools – mainly imaging tests such as CT scans with contrast administration [3]. The most common histological type of kidney cancer is clear cell carcinoma, accounting for approximately 70–80% of all kidney cancers; less common are papillary carcinoma, chromophobe carcinoma, collecting duct carcinoma, sarcomatoid carcinoma, or unclassified renal cell carcinoma [4]. Kidney cancer in its early stages does not cause any symptoms. It is estimated that more than 50% of kidney cancer cases are discovered incidentally during routine imaging tests associated with non-specific symptoms in the abdominal cavity, chest, or spine [5]. It is also estimated that approximately 15% of patients have metastases at the time of cancer diagnosis [6]. In recent years – according to the National Cancer Registry – approx. 5000 cases of kidney cancer are diagnosed in Poland each year (men – approx. 3000 cases, women – approx. 2000 cases), and approx. 2500 Poles die from this cancer each year (approx. 1500 men and approx. 1000 women) [7]. Diagnosing kidney cancer in its early stages is crucial for prognosis and potential cure. Unfortunately, no screening programs have yet been developed that could increase the early detection of kidney cancer in the population [8].
The organs to which kidney cancer most often metastasizes are the lungs, bones, and brain, but also the adrenal glands, the opposite kidney, and the liver [9]. Metastatic kidney cancer often involves distant organs and structures, which is associated with difficulties in diagnosis and treatment and, in most cases, a poor prognosis for the patient. Despite significant advances in cancer diagnosis and treatment, metastatic kidney cancer remains a serious clinical problem with a poor survival prognosis – at the highest stage, statistically only 8% of patients survive 5 years after diagnosis [10]. In addition, metastatic kidney cancer can cause a number of non-specific paraneoplastic symptoms such as hypercalcaemia, hypertension, prolonged prothrombin time, neuropathy, Cushing’s syndrome, and many others [11].
For many years, cytoreductive nephrectomy (CN) has been considered the gold standard in the treatment of patients with metastatic kidney cancer, especially in patients in good general health with no significant contraindications to surgical treatment. These assumptions were based on the results of 2 randomized phase 3 trials published in 2001, which showed that patients undergoing CN in combination with systemic interferon-α (INF-α) treatment had an increased survival rate compared to those on INF-α alone [12, 13].
The precise pathophysiological mechanisms explaining the role of CN in the treatment of mRCC remain unclear. First, the role of CN is to reduce the mass of the tumour, which eliminates symptoms such as pain, pressure on adjacent organs, or haematuria, improving the patient’s quality of life. Primary tumour resection reduces the source of tumour growth factors, stimulates the immune system response, and removes a trap for trafficking lymphocytes, increasing the chance for a better clinical response and inhibition of tumour progression [12, 13]. Furthermore, CN is believed to result in the removal of pro-angiogenic factors as well as regulate immune suppression, with a positive effect on residual disease [14]. Clinical data support that large tumours are more immunosuppressive compared to small tumours on both the local and systemic level, directly impacting the ability of the host immune system to effectively mount natural or immunotherapy-induced immune responses. Therefore, the removal of the tumour mass increases the chance for an appropriate response of the immune system and inhibition of the progression of the disease [15]. It has been observed that CN causes mild renal failure, detected in laboratory tests as an increase in creatinine levels, which usually causes mild metabolic acidosis. This induced metabolic acidosis may contribute to the reduction of tumour invasion. A disturbance of tissue pH changes the microenvironment of the tumour and surrounding tissues, thus reducing the rate of tumour growth and disease progression [16]. Importantly, metastatic kidney cancer is an immunogenic tumour that modulates the host immune response, which results in the suppression of the antitumour activity [17]. Removal of the tumour by performing CN may eliminate this problem.
In modern clinical practice, the indications for CN in patients with metastatic kidney cancer as first- or second-line treatment (after systemic therapy) are as follows:
Relief of primary tumour symptoms (such as haematuria, pain, and tumour mass effect);
Removal of the primary tumour in patients with few metastases or with complete resolution/significant reduction of metastases after systemic treatment;
Removal of the primary tumour in kidney cancer patients in the favourable prognosis group with less than one risk factor according to the International Metas- tatic Renal-Cell Carcinoma Database Consortium (IMDC) [12, 13, 18, 19].
In certain clinical situations, the CN procedure may not be appropriate or may be contraindicated. The main contraindications to CN are as follows:
Poor general condition of the patient caused by the cancer process, making it impossible to perform surgery safely;
Presence of metastases in multiple organs, in which case CN may not be of significant benefit to the patient;
Advanced renal failure – in this case, removal of the involved kidney may lead to a worsening of the patient’s overall condition;
High surgical risk – including age, comorbidities, or other factors that prevent the safe performance of surgery;
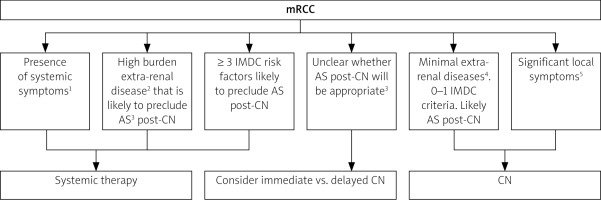
Lack of patient consent – the final decision about the procedure is made by the patient after consultation with a multidisciplinary oncology team, so lack of patient’s informed consent prevents the performance of CN (Fig. 1).
Fig. 1
Cytoreductive nephrectomy and surveillance considerations in mRCC [39]
AS – active surveillance, CN – cytoreductive nephrectomy, IMDC – International Metastatic Renal-Cell Carcinoma Database Consortium
1Fevers, chills, night sweats, etc.
2Greaterthan 20%
31–2 IMDC risk factor or ~ 15% extra-renal tumour burden
4< 10%
5Significant pain or haematuria
The use of immune checkpoint inhibitors (ICI), i.e. ipilimumab and nivolumab, in the therapy of metastatic kidney cancer has revolutionized treatment recommendations due to the high effectiveness of these drugs. Although several retrospective studies assessing the effectiveness of ICI treatment in combination with CN confirmed the role of surgical treatment in selected groups of patients, the results of CN in patients treated with ICI are currently not clearly defined. Even though the results of 2 randomized clinical trials did not support the use of CN in patients with metastatic renal cell carcinoma treated with sunitinib (cancer du rein metastatique nephrectomie et antiangiogéniques – CARMENA, and SURTIME), interest in the role of CN is renewed, and current trial results suggest that CN may still play an important role in selected patients with metastatic renal cell carcinoma treated with ICI.
Singla et al. [20] retrospectively analysed a total of 391 pa- tients with metastatic renal cancer, who were treated with ipilimumab and nivolumab, with or without CN. Of the 391 patients, 221 (56.5%) received ICI combined with CN and 170 (43.5%) received ICI alone. After 14.7 months of follow-up, patients treated with ICI combined with CN had statistically longer overall survival (OS) compared to patients treated with ICI alone, which was also confirmed in multivariate analyses. Among the patients who underwent CN, the majority (n = 197) had CN performed before ICI was initiated, compared to the second group (n = 24), in which ICI treatment was initiated first and then CN. Due to the strict criteria for including patients in the analysis, there were only 24 patients treated initially with ICI and then with CN, which significantly limited the possibility to consider the optimal time to perform CN. Importantly, in patients treated with CN combined with ICI, compared to treatment with ICI alone, there were no more frequent surgical complications of treatment, positive surgical margins, re-hospitalizations, or increases in the length of hospital stay.
In another study, Hara et al. [21] conducted a retrospective analysis of 54 patients with metastatic renal cell carcinoma treated with ipilimumab and nivolumab in combination with CN (n = 21) compared to patients treated with ICI alone (n = 33). The median follow-up time was 15.7 months. They found that the progression-free survival (PFS) was statistically longer in patients who underwent CN (10.8 months) compared to patients without CN (3.4 months; p = 0.0158). OS was significantly longer in patients who underwent CN (38.4 months) compared to patients treated with ICI alone (12.6 months; p = 0.0024). Treatment-related adverse events (grade 3 or higher) were observed in 10 patients treated with CN in combination with ICI, compared to 9 patients treated with ICI alone. There was no statistically significant difference in the occurrence of complications in the compared groups of patients, which suggests that CN is a safe and effective strategy in patients with metastatic renal cell carcinoma treated with ICI.
It should be emphasized that each case is unique, and the decision to perform CN must be well thought out and based on current medical knowledge and an individualized assessment of the patient’s condition. A multidisciplinary oncology team, including a urologist, oncologist, and psychologist, should consider all factors and potential exclusions that may constitute contraindications to surgery. Cytoreductive nephrectomy in patients with metastatic kidney cancer is associated with a higher risk of major complications and perioperative death (1.8–3.6%) than in less advanced cases [22]; therefore, it is important to refer patients to high-referral centres with experience in performing complex renal surgery.
Literature review
There are many publications in the medical literature with results that indicate a positive role of CN in the treatment of patients with metastatic kidney cancer. One of them is a 2014 paper published by Heng et al. [23]. The above-mentioned retrospective study included 1658 patients, 982 of whom underwent CN; the remaining 676 patients were treated with systemic therapy alone. The median overall survival was longer in patients with CN compared to patients without surgery, at 20.6 months versus 9.5 months, respectively (p < 0.0001). Importantly, the benefit of CN appeared to depend on the number of individual IMDC prognostic factors (Table 1). It was shown that patients with 4 or more prognostic factors according to the IMDC scale did not benefit from removal of the involved kidney. This underscores the importance of properly qualifying patients for surgical treatment based on the IMDC risk factors.
Table 1
Risk factors and prognostic categories included in the Memorial Sloan Kettering Cancer Center and International Metastatic Renal-Cell Carcinoma Database Consortium scales
| Risk factors | Prognostic categories |
|---|---|
| MSKCC scale [24] | |
| Karnofsky score < 80% Time from diagnosis to treatment initiation < 1 year Haemoglobin concentration below the lower limit of normal Corrected calcium concentration above the upper limit of normal Lactate dehydrogenase concentration above the upper limit of normal | Favourable 0 factors Intermediate 1–2 factors Poor ≥ 3 factors |
| IMDC scale [25, 26] | |
| Karnofsky score < 80% Time from diagnosis to treatment initiation < 1 year Haemoglobin concentration below the lower limit of normal Corrected calcium concentration above the upper limit of normal Neutrophil count above the upper limit of normal Platelet count above the upper limit of normal | Favourable 0 factors Intermediate 1–2 factors Poor ≥ 3 factors |
In another retrospective study, Hanna et al. analysed the efficacy of CN in patients with metastatic kidney cancer who received systemic therapy [27]. A total of 15,390 patients’ treatment histories were analysed, 5374 of whom (approximately 35%) underwent removal of a kidney involved by cancer. Median overall survival was statistically longer in patients who underwent surgery than in those who did not (17.1 months vs. 7.7 months, p < 0.001). The main limitation of the study was the inability to evaluate and retrospectively compare patients in terms of the number of IMDC prognostic factors.
Analysing the results of the above-mentioned studies on the use of CN in patients with metastatic kidney cancer, the results show a clear benefit of performing CN. This is mainly confirmed by the longer survival time of patients who underwent CN compared to patients who did not undergo surgical treatment.
The starting point for considerations questioning the validity of performing CN in patients with metastatic kidney cancer were the results of the CARMENA randomized phase 3 clinical trial, published in 2018, which evaluated the role of CN in patients with metastatic kidney cancer treated with sunitinib [28]. This drug, a tyrosine kinase inhibitor, has the ability to inhibit a number of key signalling pathways involved in the processes of cancer development and growth. It works by inhibiting angiogenesis – the formation of new blood vessels that supply blood and nutrients to the tumour – which can limit tumour growth and inhibit cancer cell proliferation. Sunitinib was approved by the U.S. Food and Drug Administration in 2006 as a first-line treatment for patients with advanced kidney cancer. The approval of the drug in this indication was based on the results of a phase 3 study in which patients treated with sunitinib had a significantly longer median PFS (11 months) than patients treated with INF-α (5 months), previously the leading systemic treatment for metastatic kidney cancer [29]. Since then, sunitinib has effectively replaced INF-α in the treatment of metastatic kidney cancer due to better clinical outcomes and higher therapeutic efficacy.
The above-mentioned CARMENA study enrolled 450 pa- tients (intermediate and poor prognosis group according to Memorial Sloan Kettering Cancer Center – MSKCC (Table 1) randomly assigned to an experimental arm (CN + sunitinib 226 patients in total) and a control arm (sunitinib only 224 patients in total). The study was designed to test whether sunitinib alone is not inferior (non-inferiority) to nephrectomy followed by sunitinib. The results were surprising: the median overall survival was shorter in patients who received CN in combination with systemic treatment with sunitinib compared to patients who received systemic treatment alone without surgery. Therefore, it was concluded that sunitinib alone is not worse than nephrectomy followed by sunitinib, thus questioning the validity of performing CN in patients with metastatic kidney cancer. The cancer du rein metastatique nephrectomie et antiangiogéniques study showed that patients in the poor prognosis group did not benefit from CN, which was previously the gold standard. Therefore, according to the results of the CARMENA study, patients in the poor prognosis group according to MSKCC should not undergo surgery but should only receive systemic treatment. The above results also apply to some patients in the intermediate and favourable prognosis groups.
The cancer du rein metastatique nephrectomie et antiangiogéniques study had many limitations, so its results should be interpreted with caution by urologists and oncologists. The first limitation of the CARMENA study is that the included patients were suitable candidates for nephrectomy in the subjective opinion of the attending urologist, and therefore the results are not generally applicable to patients with poor performance status, because CN is not recommended for such patients by design. Another limitation was the inclusion of only patients with high- and intermediate-risk disease according to the MSKCC scale, which was the scale in use at the start of the study. The Memorial Sloan Kettering Cancer Center scale was developed on the basis of INF-α efficacy data, and it differs significantly from the currently recommended IMDC scale, which is a newer scale developed on the basis of antiangiogenic drug therapy data (Table 1). Another limitation was the recruitment of a smaller group of patients than expected (450 patients instead of the originally expected 576). In addition, the recruitment of patients for the CARMENA study took a long time. As a result, the study had to be ended prematurely, raising controversy that the number of patients studied was too small for adequate statistical power. Low statistical power undermines the reliability of the results obtained. There are also concerns about the uneven recruitment of patients from the different centres participating in the study, which could have a negative impact on the reliability and transparency of the results obtained. Importantly, the study excluded patients with few metastases, who, in the era of current clinical data, are considered good candidates for CN followed by observation. In addition, the experimental arm (CN + sunitinib) had a higher percentage of locally advanced T3 and T4 stage tumours (70.1%) than the control arm (sunitinib only) (51.0%), which may have influenced the results of the study. All the above limitations of the CARMENA study mean that its reliability is currently being questioned by urologists and oncologists involved in the treatment of metastatic kidney cancer.
Another important study on the topic of CN in patients with metastatic kidney cancer treated with sunitinib is the SURTIME clinical trial conducted between July 2010 and March 2016, the results of which were published in 2019 [22]. In this randomized study, patients were randomly assigned to 2 groups. In the first group (experimental group), sunitinib treatment was started before CN and continued after CN. The second group of patients (control group) did not receive the initial treatment with sunitinib, but instead received CN followed by sunitinib. A total of 99 patients were enrolled in the SURTIME study, and treatment outcomes were compared with respect to the assumed 28-week PFS. The primary goal of the SURTIME study was to determine whether pretreatment with sunitinib prior to CN improves outcome. Another goal of the study was to identify patients refractory to systemic therapy, who are unlikely to benefit clinically from CN. Previous single-arm phase 2 studies of delayed CN after preoperative sunitinib showed that this approach is safe and helps avoid CN in people with early resistance to tyrosine kinase inhibitors (VEGFR) [21, 22, 30, 31]. In addition, the approach of delayed nephrectomy after initiation of preoperative treatment with sunitinib may reduce the size and vascularity of the primary tumour, thereby facilitating the procedure and reducing surgical and perioperative risks [32, 33].
No differences in PFS were observed between the 2 gro- ups in the SURTIME study (experimental and control). However, there was a reduction in the relative risk of death in patients in the experimental group (patients treated with sunitinib prior to CN) compared to patients in the control group. The median overall survival was significantly longer in patients treated with sunitinib prior to nephrectomy, at approximately 32.4 months, compared to the control group, in which median survival was approximately 15 months. It is also worth noting that the rate of surgical complications was similar in both groups, so there was no association of preoperative sunitinib use with an increase or decrease in the risk of perioperative nephrectomy complications. Perioperative mortality after CN in the SURTIME study is comparable to that reported in the literature [34–36].
As demonstrated in the SURTIME study, another potential benefit of delayed CN is the identification of patients with aggressive disease, who do not respond to targeted therapy and are therefore less likely to benefit from CN. In other words, the use of targeted therapy prior to CN may be a useful indicator of which patients are appropriate candidates for the procedure later. In the SURTIME study, with the exception of one patient who was ineligible for initial sunitinib treatment, all patients in the experimental group received systemic treatment, while only 40 of 46 patients (86.96%) in the control group received systemic treatment. This suggests that delaying the initiation of systemic treatment by performing CN may put some patients at risk of not receiving systemic treatment. The results of the SURTIME study suggest that the delayed CN approach, in which patients are started on sunitinib and offered nephrectomy only if their disease does not progress, may be better than performing CN upfront and then including sunitinib.
According to the results of the SURTIME study, performing CN before initiating systemic treatment puts patients at risk of not receiving systemic treatment that could otherwise extend their lives. In a post-hoc analysis of the SURTIME study, a significant number of patients who underwent CN did not receive systemic treatment – only 80% of patients who underwent the procedure received sunitinib, compared to the delayed CN arm, in which 97.7% of patients received systemic treatment with sunitinib [37]. The data presented in the above study indicate that CN reduces the likelihood of initiating systemic treatment in the future and shortens the duration of systemic treatment, while the delayed CN approach allows for better disease control and better selection of patients suitable for surgery.
Like the CARMENA study, the SURTIME study was not without limitations. First, the study did not enrol as many patients as originally anticipated, and it ended after only 99 patients with metastatic kidney cancer were qualified. In addition, the study was closed after 5.7 years, which, given the small number of patients enrolled, meant that recruitment took a very long time and was not satisfactory. It should also be noted that there was a significant discrepancy in the gender of the patients recruited: 80 men and 19 women, which is not in line with population data, according to which kidney cancer is approximately 1.5 times more common in men than in women [1].
Conclusions
For some patients with advanced kidney cancer, CN still plays an important role in treatment. However, CN is not performed arbitrarily in all patients, but only in those for whom the risk-benefit analysis leads the multidisciplinary oncology team to decide on surgery. As mentioned above, the approach to performing CN has changed dramatically with the publication of the results of the CARMENA and SURTIME prospective randomized trials. The results of the CARMENA trial show that patients with poor prognosis do not benefit from CN, while the results of the SURTIME trial suggest that initial targeted sunitinib therapy prior to CN may play a positive role in treatment.
Careful selection and appropriate qualification of patients for surgery is of great importance in the context of planning the therapeutic process and should be carried out by a multidisciplinary oncology team whose goal is to determine the feasibility of nephrectomy, assess the perioperative risks, and – above all – evaluate the benefits of surgery. If the perioperative risk is too high or if the procedure may not yield the expected results, the procedure should be abandoned. If the goal of therapy is primarily to control symptoms and maintain the patient’s quality of life rather than prolong life, the physician may decide to use only systemic treatment to reduce pain and improve the patient’s quality of life. In the process of qualifying for CN, the opinion of the patient should also be taken into account because they may not agree to surgery after being presented with the benefits and risks of surgery. The cytoreductive nephrectomy compromise is based on an individual approach to each patient, taking into account both the patient’s medical condition and the risks associated with the surgery.
According to current guidelines, patients with metastatic kidney cancer and an unfavourable prognosis according to the IMDC should not undergo CN. Patients in the favourable prognosis group according to IMDC should undergo CN if they do not require immediate systemic treatment. The decision regarding surgical treatment of patients in the intermediate group, between the 2 groups mentioned above, should be made by a multidisciplinary oncology team, taking into account possible initial systemic treatment as a “litmus test” to identify patients who may benefit from undergoing CN [38].
Removing the involved kidney may have benefits, but it also has some risks and potential side effects. Factors in favour of performing CN include the following:
Reduction of disease symptoms and improved quality of life;
Improved response to molecular targeted therapy or immunotherapy;
Control of local tumour spread.
Potential risks associated with CN are as follows:
Operative and perioperative risks;
Risk of loss of renal function resulting in the need for renal replacement therapy;
Need for postoperative convalescence;
No guarantee of the effectiveness of the procedure – CN does not always provide the expected benefits – there is a risk that the procedure may not stop the progression of the disease.
So far, few research results have been published assessing the effectiveness of CN in combination with ICI, i.e. ipilimumab and nivolumab. Due to the high effectiveness of ICI in the oncological treatment of patients with metastatic renal cancer, the strategy of combining ICI with CN is the subject of prospective clinical trials assessing the potential benefits, indications, contraindications, and the appropriate time (CN before or after ICI) for the initiation of surgical treatment. It is crucial to precisely define the groups of patients who may benefit from CN and those in whom surgical treatment should be avoided. Moreover, an important issue is to determine whether CN should be performed before or after ICI treatment; currently, it seems more beneficial and safer to perform CN after initial ICI treatment [20].
In the previously mentioned research results conducted by Singla et al. [20] and Hara et al. [21], no negative impact of ICI on surgical risk or postoperative complications was found in patients in whom ICI treatment was combined with CN. The proper selection of patients for CN is of great importance, also in the context of potential postoperative complications. The preoperative qualification will probably be improved as the results of ongoing prospective clinical trials on this topic appear. Until the clinical indications for CN are determined, the decision about the procedure should be made by a multidisciplinary oncology team.
Due to the appearance of new immunomodulatory drugs, including ipilimumab and nivolumab, which are included in the treatment of advanced-stage kidney cancer patients, the role of sunitinib has been limited. The superiority of ipilimumab and nivolumab over sunitinib has changed the guidelines for first-line treatment of patients with metastatic kidney cancer and limits the applicability of the results of the CARMENA and SURTIME trials. Despite these limitations, the results of the above studies are important in guiding treatment decisions for patients with metastatic kidney cancer who require sunitinib.
The final decision to perform CN depends on many factors, including disease stage, overall health status, and individual contraindications to surgery. When making treatment decisions, a multidisciplinary oncology team should consider the potential benefits and risks associated with surgical removal of the involved kidney.
As guidelines and recommendations for the treatment of metastatic kidney cancer continue to be evaluated, it is necessary to continually monitor and study the role of CN in contemporary kidney cancer treatment regimens. A prospective and randomized long-term evaluation of the impact of CN on the outcomes of oncologic treatment of patients with metastatic kidney in specific prognostic groups is recommended. However, when planning future studies, it is advisable to eliminate the factors described above that reduced the clinical value of the CARMENA and SURTIME studies. Importantly, future studies should aim to characterize not only the safety and efficacy profile of CN, but also the relationship between surgical treatment and patients’ quality of life.








