Introduction
Malaria is one of the most common mosquito-borne disease worldwide. In 2020, an estimated 241 million cases of malaria were reported worldwide with the number of deaths being approximately 627,000 people [1]. Almost 60% of malaria deaths worldwide occur in the poorest 20% of the population. It also imposes a major financial and social burden on many regions of the world. Monocytes are part of the immune system controlling parasite burden and protecting the host against malaria infection [2]. They play their protective roles against malaria via phagocytosis, cytokine production and antigen presentation [3].
The function of monocytes
Monocytes are a type of white blood cell derived from myeloid progenitors which play a crucial role in the innate immune system. Monocytes have two important roles in the immune system. First, they regenerate resident macrophages and dendritic cells under normal conditions, and secondly, they travel to infection sites in the tissues and differentiate into macrophages and dendritic cells to induce an immune response to inflammation signals. Monocytes kill pathogens as well as facilitating the healing and repair process [4]. They are a key component of the innate immune system serving three main immunological functions: phagocytosis, antigen presentation and inflammatory cytokine production [5].
Monocytes can phagocytose pathogens by binding to them directly through pattern-recognition receptors, or by using intermediary opsonizing proteins such as antibodies or complement, which coat the pathogen. Monocytes may also use antibody-dependent cell-mediated cytotoxicity to destroy infected host cells. Monocytes function as phagocytes and antigen-presenting cells in the peripheral blood to ingest and remove microorganisms, foreign material, and dead or damaged cells.
Microbial pattern-recognition receptors recognize pathogen-associated molecular patterns (PAMP) and activate monocytes to kill invading parasites. An example of pattern-recognition receptors is the mammalian Toll-like receptor (TLR), which recognizes a variety of microbial pathogens and their products [6]. Monocytes express more TLRs than neutrophils. When specific ligands bind, TLRs activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and stimulate the production of proinflammatory cytokine from monocytes through a pathway involving the adaptor protein MyD88 [7].
Monocytes produce cytokines, which attract more cells and proteins to the infected region, resulting in an activated immune response. In response to parasite ingestion, monocytes secrete both pro-inflammatory and anti-inflammatory cytokines as well as growth factors, which results in parasite removal and minimization of inflammation [8]. Monocytes secrete pro-inflammatory cytokines such as interleukin 1 (IL-1) and tumor necrosis factor α (TNF-α), which can activate other leukocytes and endothelial cells to a pro-adhesion, pro-migratory condition and induce secretion of vasoactive substances [9].
Lymph node-trafficking monocytes are weak antigen presenters and instead serve as antigen transporters, delivering antigens to draining lymph nodes. Other studies, on the other hand, suggest that monocytes process and present antigen similarly to classical dendritic cells (cDCs) [10]. It is likely that the methodological differences are the reason for the variation of results. Both in vivo and ex vivo studies have demonstrated that monocytes play a significant role in the presentation of antigens to T cells and the induction of particular T cell subsets.
Monocyte subsets
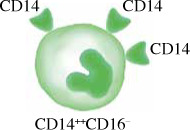
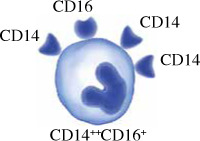
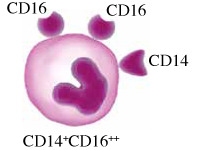
Monocytes in humans are heterogeneous. They consist of three subsets based on expression of CD14 and CD16. Circulating human monocytes consist of the CD14++CD16– classical monocytes, CD14++CD16+ intermediate monocytes and CD14+CD16++ non-classical monocytes. Classical monocytes are the most predominant subset, accounting for around 80% of the total circulating monocyte population [11]. The remaining 20% of monocytes are the non-classical monocytes and intermediate monocytes. These subsets vary in their differentiation properties, migratory capabilities, and cytokine production. However, some factors such as gender, ethnicity, age and diet may alter the proportion of monocyte subsets in individuals.
Classical monocytes
The classical monocyte is characterized by high expression of CD14. Classical monocytes were found to be primed for phagocytosis, innate sensing and migration. Classical monocytes, also known as inflammatory monocytes, have a more pro-inflammatory nature, with the ability to infiltrate tissues and produce soluble inflammatory cytokines and differentiate into DCs and inflammatory macrophages, linking between the innate and adaptive immune responses. Classical monocytes express many pattern recognition receptors (PRRs) and are involved in removing microorganisms and dying cells through phagocytosis [12].
Classical monocytes react strongly to bacterial products through TLR4 and infiltrate inflammatory sites in response to the chemokine CCL2. These monocytes proliferate in the bone marrow in response to infection or injury. For example, during bacterial infection, these monocytes migrate to the infection site, phagocytose pathogens, and produce a variety of chemokines that attract other immune cells, and present antigens through MHC class II. These monocytes may leave the blood vessels and survey the tissue microenvironment without further differentiation before exiting through the lymphatics [13]. CD14+ classical monocytes express high levels of chemokine receptors such as CCR1, CCR2, CCR5, CXCR1, and CXCR2, indicating their ability to migrate to signals arising from injured or inflamed tissues [14], but they are also distinguished by their ability to secrete pro-inflammatory molecules such as IL-6, IL-8, CCL2, CCL3, and CCL5 [15]. Classical monocytes are able to differentiate into monocyte-derived macrophages and DCs and they play an important role in regulating inflammation and tissue recovery [16].
Non-classical monocytes
Non-classical endothelial patrolling monocytes are CD14+CD16++ in humans. The non-classical monocyte shows low expression of CD14 and additional co-expression of CD16. They have high fractalkine receptor (CX3CR1) expression but they also migrate in response to a variety of chemokines [17]. Non-classical monocytes are involved in endothelium intraluminal monitoring, complement and Fc γ-mediated phagocytosis of damaged endothelium, and neutrophil recruitment to the injury site. These monocytes are able to detect and respond to circulating nucleic acids and viruses via TLR7 signaling, and trigger an innate immune response by secreting cytokines and chemokines [18]. However, the exact role of the non-classical monocytes is still debatable. They have antigen-processing abilities [19], but they differ from classical monocytes in that they are involved in wound healing proces- ses [20]. Moreover, they have antagonizing functions towards classical monocytes and promote neutrophil adhesion at the endothelial interface via the secretion of TNF-α [21] but they do not produce pro-inflammatory cytokines at the same levels as the classical monocytes.
Intermediate monocytes
Intermediate monocytes CD14+CD16+ in humans have also been identified, but their specific function is still unknown. The intermediate monocyte has high expression of CD14 and low expression of CD16. These intermediate monocytes were found to have similar ROS production and phagocytosis ability as the classical monocytes, but have lower cell surface adhesion and express a higher level of class II molecule and IL-12 [22]. Intermediate monocytes may be a transitional stage of maturation from classical to non-classical monocytes, and they react strongly to viral and bacterial ligands. Intermediate monocytes were the only subset of monocytes that expressed CCR5, making them suitable for antigen presentation, cytokine secretion, apoptosis regulation, and differentiation [23]. Their ability to present antigens and activate T cells is indicated by their gene expression signature. Based on transcriptome studies, intermediate monocytes express more MHC II and are more similar to classical monocytes than non-classical monocytes. Intermediate monocytes also have proinflammatory functions by secreting large quantities of IL-1β, IL-6, IL-12, TNF-α and CCL3 when stimulated by TLR [24]. However, their exact function in immunity is still unknown, as another study revealed that they are the primary producers of IL-10 in response to TLR stimulation [25].
The monocyte subsets show significant variability in surface marker expression and functions; however, the exact role of different monocyte subsets in malaria infection is still unclear. The intermediate and non-classical monocytes have previously been shown to play a significant role in parasitic infection. Classical monocytes have a more pro-inflammatory physiology due to their ability to produce soluble mediators and differentiate into monocyte-derived DCs to link innate and adaptive immune responses. Meanwhile, intermediate monocytes are involved in antigen presentation, whereas non-classical monocytes play a crucial role in anti-viral reactions [10].
Based on their maturation status, monocytes can be differentiated into different subsets which vary in their cytokine secretion and ability to phagocytose merozoites, or infected RBCs (iRBCs). For example, the intermediate monocyte subset appeared to be the monocyte subtype best suited for phagocytosis of P. vivax-infected cells in vitro when compared to the more mature non-classical subset. In P. falciparum infection [26], the expression of CD16/FcγRIIIa on these two subsets is associated with TNF-α production. In severe malarial anemia (SMA) in children, circulating red blood cells are known to be IgG-coated. IgG-coated red cells formed during P. falciparum infection might engage CD16/FcγRIIIa on monocytes, accelerating the destruction by erythrophagocytosis. Thus a negative correlation of the CD16/FcγRIIIa expression level with hemoglobin levels was observed in the more mature non-classical subset, indicating its role in erythrophagocytosis [27]. While there are still large gaps in our understanding of the roles of these monocyte subsets in malaria, they may have enhanced antiparasitic activity.
In regards to the secretion of inflammatory cytokines, the classical, intermediate, and non-classical monocyte subsets differ in their production of IL-1β, IL-6, and TNF-α (Fig. 1). Boyette et al. reported that the classical monocytes are the best cytokine producers and non-classical monocytes are poor cytokine producers. The level of TNF-α produced by intermediate monocytes was equivalent to that produced by classical monocytes. The classical monocyte subset secreted more IL-1β than the other subsets. Accordingly, the classical subset secreted more IL-6 than the non-classical and intermediate subsets [28].
Fig. 1
The role of monocytes in malaria infection. A) Phagocytosis. Monocytes phagocytose iRBCs and merozoites through opsonic or non-opsonic phagocytosis. B) Antibody-dependent cellular inhibition (ADCI). Monocytes interact with antibody-opsonized merozoites through FcγRs. C) Cytokine production. Parasite-associated components activate monocytes via TLRs
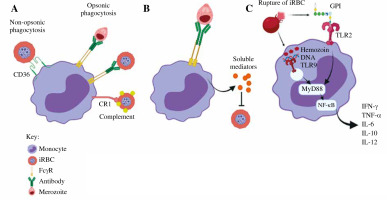
The role of monocytes in malaria
The malaria parasite interacts with the innate immune system during its life cycle, with the monocytes and macrophages playing major roles in tissue-specific inflammatory responses. During an acute malaria infection, monocytes are recruited in large numbers from the bone marrow. When monocytes migrate into tissues, these monocytes differentiate into macrophages or DCs, which improve the phagocytic and antigen presentation capabilities. Monocytes migrate through the bloodstream between the bone marrow and the target organs. Once activated, monocytes help to decrease the parasite burden by phagocytosis, cytokine production, and antigen presentation [29]. Table 1 summarizes the role of monocytes in different malaria cases.
Table 1
The role of monocytes in different malaria cases
| Malaria condition | The role of monocytes | References |
|---|---|---|
| Acute P. falciparum malaria | Monocytes increase secretion of the proinflammatory cytokines TNF-α, IP-10 (CXCL10), IFN-γ, and decrease phagocytosis of iRBCs | [11] |
| Acute P. vivax infection | Inflammatory and classical monocytes secrete inflammatory mediators, TNF-α, IL-6, and IL-8 | [32] |
| Severe malaria | Inflammatory monocyte subset increases with higher levels of proinflammatory cytokines (IFN-α, IFN-γ, TNF-α) and chemokines (CCL2, CCL3, CCL4, CXCL10) | [32] |
| Placental malaria | Opsonic phagocytosis of iRBCs by monocytes helps to eliminate iRBCs. Production of proinflammatory mediators (e.g. IL-1β) by monocytes can destroy the structure and impair the functions of the syncytiotrophoblast. Monocyte accumulation in placenta malaria causes reduced levels of insulin-like growth factor-1, a positive regulator of fetal growth. In addition, excessive monocyte activation may disrupt placental angiogenesis | [11, 32, 47] |
| Severe malarial anemia | Monocytes increased phagocytic activity, leading to accelerated destruction of infected and uninfected erythrocytes. Activation of monocytes by iRBCs led to excessive production of proinflammatory mediators in response to the uptake of erythropoietin, such as TNF-α and nitric oxide, which are associated with suppression of erythropoiesis in the bone marrow. Monocytes loaded with hemozoin suppress erythropoiesis in the bone marrow by inducing apoptosis of the erythroid progenitors via IFN-γ | [32, 47, 54, 55] |
| Cerebral malaria | Monocytes can be activated by platelet factor-4 (PF4) to produce reactive oxygen species that subsequently promote endothelial cell apoptosis. Monocytes promote a procoagulant cascade, resulting in increased expression of adhesion molecules and secretion of cytokines such as IL-2, IL-6, IL-8, IL-10, TNF-α, and IFN-γ, which initiate dysregulated hemostasis in cerebral malaria | [47, 54] |
| Acute lung injury/acute respiratory distress syndrome (ALI/ARDS) | Activation of monocytes results in the secretion of pro-inflammatory cytokines such as IL-1β and IL-18. These cytokines upregulate adhesion molecules in lung endothelial cells to promote both iRBCs and monocyte sequestration, which lead to increased vascular permeability and cause local injury via inflammatory mediators | [47] |
Monocytes can be activated directly or indirectly when various parasite components activate other host immune factors. When merozoites invade RBCs, significant changes occur to the surface of the iRBC, including the addition of parasite proteins such as Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) [30] that interact with monocyte surface receptors [31]. iRBCs also result in complement activation, leading to the deposition of C3b fragments on the iRBC surface and communication with monocyte/macrophage complement receptors. Antibodies specific for iRBC surface antigens can be used to recognize iRBCs through monocyte and macrophages Fcγ receptors (FcγRs). Merozoites are released from the iRBCs when iRBCs rupture; they are then attacked by antibodies and activate monocytes/macrophages through FcγR interactions [32]. Monocytes also have surface and intracellular Toll-like receptors (TLRs), which help recognize pathogen- associated molecular patterns (PAMPs). PAMPs that can directly activate TLRs expressed by monocytes/macrophages including glycosylphosphatidylinositol (GPI) anchors, Plasmodium DNA, RNA and hemozoin crystals [33].
Phagocytosis
Monocytes are unable to phagocytose RBCs infected with mature gametocytes [34], but their ability to phagocytose merozoites and asexual iRBCs is crucial for parasitemia regulation. A previous phagocytic assay performed in vitro revealed that intermediate CD14++ CD16+ monocytes were the most effective subset for phagocytosis of Plasmodium vivax iRBCs and complement opsonized P. falciparum iRBCs. In malaria-infected individuals, monocytes are recruited for merozoite phagocytosis by opsonizing antibodies against MSP1 [35], while cytophilic immunoglobulins (IgG1 and IgG3) against MSP2 and MSP3 activate monocytes [36].
Monocytes play an important role in the host’s protection against P. falciparum infection, including non-opsonic and opsonic phagocytosis of iRBC (Fig. 1). During the early phase of malaria infection, monocytes bind and engulf iRBC via the CD36 receptor by non-opsonic phagocytosis. The adherence of iRBCs to CD36 is facilitated by PfEMP-1. Proteolytic degradation of PfEMP-1 from iRBCs decreased the phagocytic activity, suggesting the key role of CD36-mediated adhesion in non-opsonic phagocytosis of iRBCs whereby pharmacological inhibition of CD36-dependent signaling reduces iRBCs uptake, though there is a possibility that additional receptors are also involved. Monocytic CD36 uses both the ERK and p38 MAPK signaling cascades to actively participate in the non-opsonic phagocytosis of iRBCs. However, opsonic phagocytosis leads to more iRBC uptake than the non-opsonic process. Opsonic phagocytosis involves the interaction between opsonin and specific receptors. When the complement system is activated, C3b and C4b act as opsonin which target antigens for phagocytosis. The complement-bound parasite antigens bind with complement receptors on monocytes, and they are subsequently phagocytosed by monocytes [37]. Furthermore, specific antibodies which function as opsonins are produced to facilitate phagocytosis by binding to FcγRs such as FcγRI (CD64), FcγRII (CD32), and FcγRIIIa (CD16). Binding of antibodies to pathogen results in complement deposition and Fc-receptor-mediated phagocytosis. Since various immune cells express different subsets of FcγRs, the efficacy of antibody binding to FcγRs varies depending on the isotype and the immune cell to which the antibody binds. The high-affinity receptor FcγRI can bind monomeric forms of IgG1, IgG3, and IgG4. FcγRII and FcγRIIIa are low-affinity receptors that only interact with IgG in complexed or aggregated form. The FcγRIIa subtype is expressed on neutrophils and monocytes and it initiates phagocytosis, ADCC and cellular activation [38]. Monocyte phagocytic activity is increased by Plasmodium-specific IgGs, which corresponds with protection and lowers the chance of developing symptomatic malaria. Monocytes could be recruited by opsonizing antibodies for merozoite phagocytosis against merozoite surface proteins (MSP)-1, whereas cytophilic immunoglobulins (IgG1 and IgG3) against MSP2 and MSP3 effectively activate monocytes [39]. Human antimalarial antibodies are naturally acquired in the body to prevent merozoite invasion of red blood cells during blood-stage malaria infections through a process known as antibody-mediated complement-dependent inhibition (ADCI). C1q fixation has been found to be the main mediator of ADCI inhibition, and the main targets are MSP-1 and MSP-2 [40]. When the parasites bind to monocytes, the receptor–parasite complex is phagocytosed by an actin-dependent mechanism. The phagosome fuses with lysosomes to form phagolysosome and the parasite components are degraded by acidic proteases.
Hemozoin is released during schizont rupture and immediately phagocytosed by monocytes, macrophages, and DCs [41]. The phagocytosis of hemozoin induces monocytes to undergo oxidative burst and downregulation of MHC class II, intercellular adhesion molecule 1 (ICAM-1), and CD11c expression [42]. Additionally, in co-culture experiments by Kumsiri et al., iRBC or hemozoin has been reported to induce production of B cell activation factor (BAFF) by monocytes, which may promote antibody production [43]. According to Bobade et al., in vitro phagocytosis of hemozoin by monocytes increases the production of IL-10, chemokine ligand 1 (CCL1), CCL17 and the expression of mannose binding lectin receptor (CD206) [41].
Antibody-dependent cellular inhibition
Merozoite surface antigens which are released during schizont rupture induce monocyte-mediated antibody-dependent cellular inhibition (ADCI) of Plasmodium falciparum provided merozoites are opsonized with cytophilic antibodies (IgG1 and IgG3) subtypes [44]. After being exposed to opsonized merozoites in vitro, monocytes release soluble mediators that inhibit parasite growth in iRBCs [45]. The interaction of IgG-merozoite complexes with monocyte FcγRs is needed for ADCI as shown in an in vitro functional assay. An earlier finding was that blocking FcγRI does not affect ADCI, but blocking either FcγRII or FcγRIII could terminate ADCI [46]. The minor CD16+ monocyte subset is needed to enhance ADCI. More research is needed to determine how monocytes can be differentially activated from macrophages in their ability to initiate ADCI and which inflammatory mediators released by monocytes suppress the growth of the parasite [47].
Cytokine production
In response to Plasmodium infection, monocytes secrete pro-inflammatory cytokines, which helps to inhibit parasite growth and infection clearance, but excessive production contributes to pathogenesis. Glycosylphosphatidylinositols (GPIs) are abundantly expressed on the parasite surface monocytes. These malarial GPIs stimulate a pro-inflammatory response by increasing the secretion of TNF-α, IL-1β, IL-6, IL-12, and nitric oxide (NO). Monocytes have been shown to produce large amounts of IL-12 and IL-18 that prevent progression to severe malaria during early infection. IL-18 and IL-12 work together to activate interferon γ (IFN-γ), which is necessary for the activation of monocyte proinflammatory function in order to facilitate parasite clearance [48]. Upon in vitro exposure to P. falciparum, production of GM-CSF, MIP-1β, or IL-34 cytokines initiates the immunity mechanism with lower parasite loads (premunition) by opsonic phagocytosis and cytokine secretions by monocytes [49]. Exposure of these cytokines, specifically TNF-α and IFN-γ, regulates iRBC uptake and endothelial cell activation by increasing the expression of ICAM-1 and other adhesion molecules by endothelial cells [50].
During blood-stage infection with P. falciparum, the inflammatory monocytes increase the expression of activation markers HLA-DR and CD86, which are involved in T cell priming [51]. Stanisic et al. demonstrated that monocytes in children with severe malaria secreted more TNF-α, MIP-1β, and MIP-1α, which are involved in monocyte activation and recruitment than healthy children or children with uncomplicated malaria [52]. The severity of the infection is thought to be influenced by the balance of pro-inflammatory and anti-inflammatory cytokines, chemokines, growth factors, and effector molecules (Table 2). It has been shown that several cytokines such as IL-1β, IL-6, IL-8, and TNF-α are increased in late-onset severe infection [53]. However, the exact function of these cytokines remains unclear and further investigation is required.
Table 2
Characteristics of monocyte subsets. The table shows comparisons between monocyte subsets in terms of their frequency in blood, surface markers, their roles and cytokine production
Other myeloid cells involved during malaria infection
Macrophages
Mature macrophages are derived from monocytes, stem cells, or from the cell division of pre-existing macrophages [56]. Macrophages lack granules but are densely packed with lysosomes. Macrophages are involved in the clearance of iRBC and control of parasitemia, whereby deficiency of monocytes and macrophages has been shown to accelerate parasite growth and anemia [57]. Infected RBCs can be phagocytosed by macrophages via two different mechanisms. The first mechanism does not involve opsonizing antibodies. Macrophages bind to antigens of the parasites that are expressed on iRBCs through surface receptors after being activated by proinflammatory cytokines such as TNF-α and IFN-γ. For instance, in human malaria, the scavenger receptor CD36 binds to the P. falciparum erythrocyte membrane protein-1 (PfEMP-1). Macrophages are also necessary for parasite clearance during adaptive immunity when the second mechanism, antibody-dependent phagocytosis, takes over [58]. In malaria-immune individuals, antibody-opsonized iRBCs and merozoites are phagocytosed by macrophages via FcγRs [59]. Other than spleen resident macrophages, CD11bhighLy6C+ monocytes have been reported to actively participate in the control of acute parasitemia in murine.
Dendritic cells
Dendritic cells are antigen-presenting cells that engulf pathogens, playing a crucial role in both innate and adaptive immune responses. This is mainly attributable to their presence at pathogen entrance points, their specific ability to sample, uptake, process, and present antigens, and their ability to integrate and react to signals from microbial and other immune cells. Dendritic cells consist of plasmacytoid [CD123+ CD11c–, (pDC)] and myeloid/conventional [CD123– CD11c+, (cDC)] [60]. Though pDCs are most often associated with viral infection defense, they express high levels of TLR7 and TLR9 and can be a major source of type 1 IFNs in other infections [61]. On the other hand, following parasite infection, monocytes are infiltrated and differentiate into MoDC [62].
Dendritic cells express a wide range of PRR on their surface, such as TLRs, which enables them to communicate with diverse microbial molecules. During blood stage malaria, DCs in the spleen monitor the blood flowing through the marginal sinus, and once activated, they can migrate to the white pulp, where they initiate acquired immune responses. A previous study demonstrated that monocyte-derived DCs could communicate with P. falciparum iRBCs by binding of CD36 to PfEMP-1 [63].
After being stimulated, DCs migrate to draining lymph nodes, where they present antigen via MHC class I or II complexes, followed by costimulatory signals such as CD40, CD80, and CD86 to CD4+ and CD8+ T cells. The antigen-MHC I or MHC II complexes are recognized by naïve CD4+ and CD8+ T cells, which proliferate and differentiate into effector cells. Human DCs effectively phagocytose P. falciparum-infected RBCs in vitro; however, the activation depends on the dose. A high iRBC : dendritic cell (iRBC : DC) ratio suppresses maturation by inducing apoptosis and blocking LPS stimulation [64]. At a 3 : 1 iRBC : DC ratio, there is an increase in the expression level of maturation markers such as HLA-DR, CD80, CD86 and CD40, as well as chemokines including CCL2, CXCL9 and CXCL10 [65]. At a 1 : 3 ratio of iRBCs, DCs induce an antigen specific T helper 1 (Th1) cell response, leading to T cell proliferation and secretion of IFN-γ, IL-10 and TNF-α. This also increases the production of type 1 IFN, CXCL9 and CXCL10, which play a role in the induction of type I regulatory T cells (Tr1). Type 1 IFNs have been shown to suppress the production of parasitic-specific CD4+ T cell IFN-γ and enhance Th1 or Tr1 cells, despite the fact that they induce an antiviral immune response. Type 1 IFNs also inhibit the production of IL-6, which impairs the ability of blood monocytes to induce inflammatory reactions [66].
During early malaria infection, parasites induce DCs to produce TNF-α and IL-12, which then activate IFN-γ. As the disease progresses, DCs produce less IL-12 and start to produce IL-10. At the later stage of infection, activated DCs are resistant to TLR stimulation, reducing their ability to phagocytose antigens and priming T cells [67]. A previous study reported that a high dose of P. falciparum iRBCs induces apoptosis in monocyte-derived human DCs while low doses trigger them to induce CD4+ T cell proliferation. Therefore it has been suggested that CD8+ cDCs, which are the major producers of IL-12, may be vital in early infection to activate Th1 responses, whereas CD8-cDCs may play a major role during the acute phase to switch from Th1 to Th2 immune responses [68].
Importance of trained immunity in malaria
The “trained immunity” phenotype relates to a prime condition that increases reactivity of monocyte and macrophages to a secondary challenge after the first stimulus. This phenotype involves epigenetic modifications, metabolic remodeling or cytokine production. TLRs and other pattern recognition receptors are believed to help monocytes in developing a Plasmodium-specific memory.
In humans, exposure of radiation-attenuated sporozoite elicits both antibody and T cell responses against sporozoite and blood-stage antigens and this protection has been associated with pluripotent effector memory T cells that produce IFN-γ, TNF-α and IL-2 that are targeted against liver rather than blood-stage antigens [69]. Jacob and colleagues found that following a secondary TLR ligand exposure, the malarial parasite and its crystal hemozoin may evoke trained immunity as indicated by inflammatory gene expression. Despite the fact that these two stimuli have comparable effects on the inflammatory transcriptome, the differential regulation of iRBC- and hemozoin-induced training by known trained immunity inhibitors suggests that the two stimuli have different training mechanisms.
Monocytes that have been “trained” or primed produce more proinflammatory cytokines when they are challenged to a secondary stimulus [70]. Recent studies have demonstrated a priming effect during P. falciparum infection on the innate immune system. McCall et al. reported stimulated TLR4 and TLR2/TLR1 responses during sub-patent blood-stage infection, which later normalized following curative treatment using an experimental human malaria model [71]. Furthermore, Franklin et al. showed that PBMCs from Brazilian adults with uncomplicated falciparum malaria had primed innate responses to numerous TLR ligands, including TLR4 and TLR2, and stronger proinflammatory cytokine responses than those seen in the experimental malaria volunteers who experienced reversed TLR responses after treatment [72]. Repeated clinical malaria infections may have a training effect on monocytes that lasts for several weeks. Increased extracellular heme and proinflammatory cytokines, such as IP-10, associated with mouse malaria models and human P. falciparum infections have also been reported to increase expression of TLRs, such as TLR4, on the surface of monocyte and macrophages and circulating endothelial progenitor cells [73].
Concluding remarks
Malaria remains a significant global health concern. Monocytes respond to malaria in many different ways, such as by phagocytosis, cytokine secretions and antigen presentation, which can be either protective or pathologic. With substantial phenotypic and functional variations among monocyte subsets, the mechanism of immune responses induced by monocytes remains complicated, and the interaction of the different possible responses is a key factor in deciding disease outcome. In future, it is necessary to focus on investigating how these monocytes are regulated differently depending on the disease severity and different Plasmodium species, the mechanisms involved in recognition of malaria and effects of disease on monocyte function. The knowledge obtained can be useful in the development of antimalarial therapy by modulating the function of monocytes in malaria, giving a new opportunity to develop novel therapeutic strategies.