Introduction
In 2023, it has been 60 years since the first pacemaker was implanted in Poland. The first defibrillator was implanted in Poland in 1986, the first cardioverter defibrillator (ICD) with transvenous electrodes was implanted in 1995, and the first resynchronization therapy pacing device was implanted in 1997. The number of implantable cardiac electrotherapy devices (CIEDs), including pacemakers, cardioverter-defibrillators, and resynchronization therapy systems, has been systematically increasing; for about 5 years, the number of procedures per 1 million inhabitants has been stable and in line with clinical needs, which is above the European average. Nowadays in Poland, approximately 750 pacemakers and approximately 250 defibrillation devices are implanted (de novo implantation or replacement) per million inhabitants per year, i.e. approximately 38,000 devices per year. It is estimated that nearly 500,000 Poles have an implanted cardiac electrotherapy device, making optimal diagnostic imaging with the use of magnetic resonance imaging (MRI) a clinically and epidemiologically important issue.
MRI is an imaging method that was introduced to medicine in the 1970s. Originally, this method, apart from the high cost, had many limitations, which narrowed the scope of its application. Currently, thanks to the dynamic development of new techniques in MRI, it has been possible to significantly shorten the acquisition time and eliminate most artefacts such as motion artifacts (e.g. respiratory artifacts, artifacts resulting from the work of the heart or pulsating blood flow in arterial vessels). Thanks to this, it became possible to obtain good quality images not only of the central nervous system or the musculoskeletal system but also of the heart, abdominal organs, or mediastinum. Compared to other imaging tests, MRI is a comprehensive method; it enables simultaneous structural (morphological) and functional evaluation, which makes it indispensable in many fields of medicine, such as oncology, neurology, neurosurgery, cardiology, orthopaedics, and gynaecology.
MRI is widely available. This examination is recognized as the gold diagnostic standard in many disease states. In this situation, it is believed that 50-70% of patients who have a cardiac electrotherapy device may have indications for MRI examination later in life.
MRI, compared to computed tomography, is characterised by a slightly lower spatial resolution and a much larger scale of applicable precautions or contraindications to the examination. For many years, these contraindications included patients with an implanted cardiac electrotherapy device. It should be emphasised that at present, as a result of many years of experience, both global and Polish [1-10], confirming the safety of such procedures, MRI is possible in the vast majority of patients with CIED if certain procedures and precautions are followed.
Magnetic resonance imaging in patients with CIED according to the European Society of Cardiology guidelines
In 2013, the European Society of Cardiology (ESC) guidelines on cardiac pacing and resynchronization therapy were published, in which MRI examinations in a clinically justified situation and while maintaining appropriate procedures were awarded the class of recommendations IIb with the level of evidence B for old-type systems and IIa for systems certified by manufacturers as MRI conditional [11].
In the latest ESC guidelines published in 2021, the re-commendation to perform MRI examinations was placed a class higher and ranked in class IIa for old-type devices, and in the case of devices with manufacturer approval to perform examinations the recommendation is in the highest class of recommendations, i.e. class I with the highest quality evidence level A [12].
It should be emphasised that almost all currently implanted devices have the manufacturer’s approval to perform MRI (MRI-conditional) examinations. Most systems are approved by manufacturers for diagnostics in MRI devices with a magnetic induction of 1.5 Tesla and with the specific absorption rate (SAR) limit for the whole body < 2 W/kg (< 3.2 W/kg for the head). Some devices are also approved for 3T MRI tests with a SAR limit of < 4W/kg for the entire body. The latest guidelines of the European Society of Cardiology on cardiac pacing and resynchronization therapy in 2021 limit the number of contraindications to perform this test.
The latest guidelines of the European Society of Cardiology on cardiac pacing and resynchronization therapy from 2021 allowed a reduction in the number of contraindications to perform this test.
Principles of performing magnetic resonance imaging examinations in patients with an implanted cardiac electrotherapy device
When making the decision to refer a patient for an MRI examination, the risk-benefit ratio of this examination should be considered. Regardless of the type of implanted system, when choosing the type of diagnostic test, it is worth taking into account the presence of image artefacts resulting from the presence of a metal implant in the patient’s body. Thoracic, shoulder, and heart tests can contain artifacts that make exam evaluation difficult. How-ever, if it is not possible to choose other diagnostic methods, a magnetic resonance scan can be performed or attempted if it does not pose a high risk to the patient.
During an MRI scan in a patient with an implanted electrotherapy system an increase of lead temperature (especially at the tips), inhibition of the pacing, change in pacing mode, loss of set parameters, software, or reset of the device (to factory or rescue pacing parameters) may occur. In the case of a patient with an implantable cardioverter-defibrillator entering the gantry without first deactivating the device, inadequate shock devices may occur.
In light of the current state of knowledge, patients with epicardial leads are at highest risk of performing this test, due to the consequences of heating the system components (leads placed extravascularly). MRI in patients with abandoned, inactive electrodes is also high risk. In these patients, examination should be performed only with absolute vital indications, while ensuring the best possible protection in terms of the appropriate number of trained personnel and equipment. There is single evidence from conducted studies describing the lack of complications associated with this type of examination [6,7].
Experiences from single case reports and studies conducted on small groups are consistent with those of the authors of this statement; however, extreme caution is re-commended with this type of research as well as a strict assessment of the potential benefits of conducting this type of research due to the lack of sufficient evidence showing this method as safe, and because it potentially has the highest risk of complications due to lead heating.
There is moderate risk associated with performing MRI scans in patients with a recently implanted system (< 6 weeks). In this group of patients, examinations may be performed only in a situation where postponing the examination could be associated with the risk of health complications exceeding the risk associated with the MRI examination.
The potentially increased risk applies to patients with previous-generation systems that are not MRI certified by the manufacturer (or for patients with systems composed of components approved by the magnetic field of different manufacturers) and in the end-of-life phase of the battery. In any case, examinations in pacemaker-dependent patients should be treated with caution.
Regardless of all the above-mentioned circumstances, the MRI examination itself is always burdened with a small but real risk related to the change of pacing parameters. For the duration of the test, in most systems, it is possible to choose between switching off the pacing function (risk of bradyarrhythmia during the test) or asynchronous pacing (risk of causing tachyarrhythmia, including ventricular fibrillation due to the coincidence of pacing with intrinsic heart work). In patients with a cardioverter-defibrillator, the device’s tachyarrhythmia detection and therapy function should always be turned off before the examination, which entails the need to constantly monitor the patient and provide access to an external defibrillator.
It should be remembered that despite the evidence of the above-mentioned risks, an MRI examination in patients with an implanted electrotherapy system is an examination with a high degree of safety – with proper rules and preparation, complications are extremely rare and concern less than 1% of the examinations performed. The most common complication is a reset of the device software – in the case of systems that are not certified by the manufacturer to perform an MRI examination, the frequency of occurrence has been estimated at 0.4-0.6%; however, most often this reset is reversible after performing device interrogation after the examination – patients do not require premature replacement of the device for this reason [7,8].
In the cited studies, no fatal complications resulting from the MRI examination in a patient with an implanted device were observed. Most of the complications described in the publications concerned single cases of patients in whom the device was incorrectly reprogrammed for the duration of the examination (complications resulting from disturbed operation of the implanted system – inadequate interventions, device reset, loss of connection with the programmer) or the medical staff were not aware that the patient had an implanted device and performed the test without properly reprogramming the pacemaker or ICD.
Each time before the examination, a full follow-up of the system should be performed to confirm its proper functioning (in particular, no signs of fracture or dislocation of the electrodes), assess the condition of the battery and the patient’s pacing dependence, and select the appropriate pacing mode for the duration of the examination (if the pacing function is maintained for the duration of the examination, it is necessary to take into account the higher amplitude and width of the pacing pulse, so that despite the heating of the lead tips the pacing is effective). After the examination, another follow-up is necessary to restore the permanent pacing parameters. Figure 1 shows the proposed flow chart of patient qualification for the MRI examination along with the suggested handling of the device during the examination.
Figure 1
Recommended procedure for MRI examinations in patients with implanted cardiac electrotherapy devices
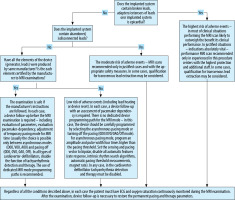
During imaging, the patient must have ECG and oxygen saturation continuously monitored. The laboratory should be equipped with a defibrillator with the function of transcutaneous pacing.
The technical conditions for conducting the tests (in terms of magnetic field inductance limits, specific absorption rate values, and gradient growth rates or possible exclusion areas) are available on the websites of the manufacturers of cardiac implantable devices (they vary depending on the manufacturer, type and model of leads, and the model of the pulse generator).
From a technical point of view, such an examination is possible in any hospital where, as well as a MRI laboratory, there is a laboratory for the control of implantable devices.
Organisation of MRI examinations
Undoubtedly, conducting such an examination safely requires increased workload, appropriate equipment, and the involvement of additional personnel. In addition to the standard team of the MRI laboratory, electrophysiological consultation and supervision is necessary, a team consisting of a cardiologist and a cardiac technician or a nurse experienced in programming devices (depending on possessed powers and competences), in order to conduct qualifications to perform the test, follow-up of the device before and after the test, adjust the pacing parameters of the device for the duration of the test, and supervise the recording of the patient’s ECG and saturation during the test [13].
In some cases, the cost of performing such an examination may also be increased by the costs of transporting the patient between the centre where the patient is hospitalized and the hospital in which the device will be checked and the imaging examination will be performed. Taking into account the factors described above, it seems necessary to provide special funding for MRI in patients with an implanted cardiac electrotherapy device by the National Health Fund.
Despite all the risk and organisational factors described above, it seems that for many hospitals, the organization and development of internal procedures to carry out such tests is achievable and should be implemented immedia-tely. A second important issue is the need for dedicated financial support for these procedures from public health insurance.






