Introduction
Psoriasis is an immune-mediated chronic inflammatory disease that co-occurs with systemic and metabolic diseases [1]. Clinical manifestations primarily manifest as psoriasis-like scales and erythema, with exacerbations frequently observed during winter months. This condition can affect various parts of the body, including the limbs and scalp, significantly impacting both the physical and mental well-being of patients. In recent years, the incidence of psoriasis has been increasing, and psoriasis vulgaris is the most common type. Its refractory and recurrent nature has attracted wide attention. It was confirmed that interleukin (IL)-17 acts in occurrence and development of psoriasis [2, 3]. IL-17 is mainly secreted by Th17 cells, and can stimulate other cells (such as keratinocytes, epidermal cells, and endothelial cells) to produce proinflammatory cytokines and chemokines [4]. The overproduction of these cytokines and chemokines leads to the formation of the detected and persistent inflammation. Second, IL-17 induces inflammatory responses in multiple cell types by binding to its receptor. In patients with psoriasis, the level of IL-17 is visibly increased, especially in the area of skin lesions [5]. High expression of IL-17 can trigger abnormal proliferation and keratinization of epidermal cells, rendering formation of psoriatic skin lesions [6]. In addition, IL-17 is also involved in regulating the activation and chemotaxis of immune cells and enhancing the infiltration and activation of inflammatory cells. IL-17 can also induce the production of IL-6, IL-8, and tumor necrosis factor (TNF)-α, which further aggravates the inflammatory response [7].
Drug therapy can enhance clinical symptoms of patients and reduce inflammatory response. Commonly used drugs for psoriasis treatment include tazarotene betamethasone ointment. Tretinoin is the active form of vitamin A, which can affect the proliferation and differentiation of keratinocytes, which is beneficial to reduce the phosphorus debris and thickness of the skin surface of patients with psoriasis [8, 9]. However, the effects of tretinoin in the treatment of psoriasis are quite variable, and it may take longer to see significant improvements. In addition, tretinoin may also cause side effects such as dry skin, irritation, and sensitivity. Secukinumab stands as one of the pioneering fully human IL-17 inhibitors, which is employed in the treatment of inflammatory conditions, particularly moderate to severe psoriasis [10]. Secukinumab was officially approved by the US Food and Drug Administration for treating psoriasis in 2015. It was approved for the therapy of moderate to severe psoriasis (MSP) in China in 2019. Secukinumab reduces inflammation, redness, swelling, and scaling in psoriatic lesion areas by inhibiting the action of IL-17A cytokines, ultimately improving the skin symptoms of patients [11]. However, there are relatively few studies on the clinical effect of tretinoin combined with secukinumab in MSP and its outcome on patients’ metabolism.
Aim
This article compared the clinical effects of tretinoin, secukinumab, and tretinoin plus secukinumab in MSP vulgaris, and detected their effects on glucolipid and uric acid (UA) metabolism, T lymphocyte subsets, liver enzyme indexes, and inflammatory factors (IFs). It aimed to provide the reference for the clinical therapy of psoriasis.
Material and methods
General information
The clinical data of 135 patients with moderate or severe psoriasis vulgaris who were hospitalized in the Central People’s Hospital of Zhanjiang from March 2021 to March 2023 were collected. Regarding the order of admission, the patients were enrolled into Tretinoin, Secukinumab, and Combination groups, with 45 cases in each. There were 30 men and 15 women in the Tretinoin group, aged 18 to 50 (35.6 ±6.3); the disease duration was 2 to 22 years (5.9 ±3.0). There were 32 men and 13 women in the Secukinumab group, aged 18 to 48 (36.1 ±6.5); the disease duration was 2 to 20 years, averaging 6.1 ±2.7 years. There were 29 males and 16 females in the Combination group, aged 18 to 50 (36.3 ±5.5); the disease duration was 3 to 20 years, averaging 6.2 ±2.4 years. The general data were similar among the subjects (p > 0.05). The trial was approved by the Ethics Committee of the Central People’s Hospital of Zhanjiang, and all subjects signed the informed consent.
Inclusion and exclusion criteria
Inclusion criteria: (1) patients conforming to the Diagnostic Criteria of the Guidelines in Moderate to Severe Psoriasis Vulgaris, 2018 edition; (2) psoriasis area and severity index (PASI) score ≥ 10, and the area of skin lesion ≥ 13%; (3) no use of immunosuppressants, corticosteroids, tretinoin, secukinumab, and other drugs within 1 month before treatment; (4) patients who voluntarily participated in this experiment.
Exclusion criteria: (1) severe infection or immunodeficiency; (2) hepatitis B, hepatitis C, syphilis, acquired immunodeficiency syndrome, active tuberculosis, and other infectious diseases; (3) malignant tumours; (4) severe heart, liver, kidney, lung, and other important viscera dysfunction; (5) blood glucose, blood lipid, UA, and other metabolic diseases and family history; (6) erythrodermic psoriasis, pustular psoriasis, and other types of psoriasis; (7) treatment drug allergy; (8) treatment cycle was shorter than 6 months.
Treatment options
In the Tretinoin group, participants took Tretinoin tablets (20 mg each, totaling 10 tablets; H10970053; LongFine Pharmaceutical Co., LTD.) at a dosage of 10 mg per administration, thrice daily. Conversely, participants in the Secukinumab group received subcutaneous injections of Secukinumab in the upper arm medial region at weeks 0, 1, 2, 3, and 4. Each injection contained 1 ml of solution at a concentration of 150 mg (Registration number S20190023). Novartis Pharma Stein AG, subpackaged by Novartis Pharmaceuticals Co., LTD., Beijing) at a dose of 300 mg per injection (divided into 2 injections of 150 mg each), followed by maintenance doses every 4 weeks. Subjects with the combination therapy were treated with oral tretinoin tablets and subcutaneous secukinumab injection at the same time points as in the Secukinumab group. The total observation period was 16 weeks.
Observation indicators
(1) Clinical efficacy: PASI score was measured before intervention and 16 weeks after intervention, and clinical efficacy was evaluated according to the improvement rate of PASI score. PASI improvement rate = (score pre-treatment-score following the treatment)/score pre-treatment × 100%. PASI improvement by 50%, 75%, 90%, and 100% were marked as PASI50, PASI75, PASI90, and PASI100, respectively, including PASI90 as the main evaluation index.
(2) T lymphocyte subsets: 3 ml fasting venous blood was collected before intervention and 16 weeks after intervention. After centrifugation, the serum CD3+, CD4+, CD8+ levels were detected by flow cytometry, and the CD4+/CD8+ index was calculated. CD3, CD4, and CD8 cell counting kits (flow cytometry) were purchased from Chengdu Nuohe Biotech Co., LTD.
(3) Metabolic indicators: serum samples were collected before intervention and 16 weeks after intervention. The levels of triglyceride (TG), total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), fasting blood glucose (FBG), and UA were detected by BIOBASE BK-600 automatic biochemical analyser.
(4) The liver enzyme indicators: serum samples were collected prior to intervention and 16 weeks post-intervention. The biochemistry analyser was applied for detection of liver enzyme indicators: alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) levels.
(5) IFs: serum IL-2, IL-6, IL-23, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) were detected by the enzyme-linked immunosorbent assay (ELISA) prior to intervention and 16 weeks post-intervention. ELISA kits were purchased from Shanghai Beyotime Biotechnology.
(6) Adverse reactions: the probability of adverse reactions after medication was observed.
Results
Contrast of PASI improvement among patients
As illustrated in Figure 1 A, after 16 weeks of intervention, subjects’ Psoriasis Area and Severity Index (PASI) scores were significantly lower compared to pre-treatment levels. The PASI scores of the Secukinumab group and subjects receiving the combination therapy were notably lower than those in the Tretinoin group. Additionally, the PASI scores of subjects in the Combination group were significantly lower than those of subjects receiving Secukinumab alone (all p < 0.05).
Figure 1
A – Contrast of PASI scores. B – PASI improvement rate; As against the same group pre-treatment, *p < 0.05; As against Tretinoin, #p < 0.05; ^p < 0.05 as against Secukinumab
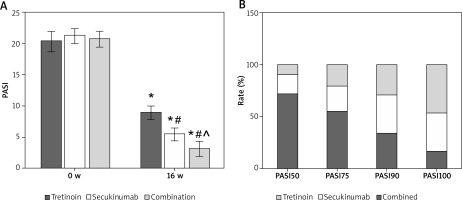
Throughout the 16-week intervention period, 12, 25, and 30 subjects achieved PASI100 in the Tretinoin, Secukinumab, and Combination groups, respectively. The proportion of subjects achieving PASI100 in the Combination group was significantly higher compared to both the Tretinoin and Secukinumab groups (Figure 1).
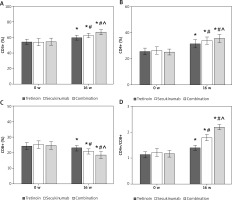
Contrast of T lymphocyte subsets levels of subjects
Over the 16 weeks of intervention, the serum levels of CD3+, CD4+, and CD4+/CD8+ ratios in subjects were significantly elevated compared to pre-treatment, while the CD8+ ratio showed a noticeable decrease from the pre-therapy levels. Relative to the Tretinoin group, both the Secukinumab group and subjects receiving the combination therapy exhibited significantly higher serum CD3+, CD4+, and CD4+/CD8+ ratios and lower CD8+ ratio. Moreover, in comparison to the Secukinumab group, subjects in the Combination group demonstrated significantly higher serum CD3+, CD4+, and CD4+/CD8+ ratios, along with a visibly lower CD8+ ratio (all p < 0.05) (Figure 2).
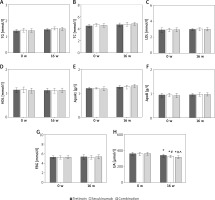
Contrast of glucose, lipid, and UA metabolism indexes of subjects
As displayed in Figure 3, there were not obviously different in serum TG, TC, LDL, HDL, ApoA1, ApoB, and FBG levels among subjects prior to the therapy (p > 0.05). The serum UA of subjects was significantly lower than prior to the therapy, and those were markedly lower in the Secukinumab group and subjects with the combination therapy as against the Tretinoin group. The serum UA level of subjects with the combination therapy was significantly lower relative to subjects with Secukinumab (all p < 0.05).
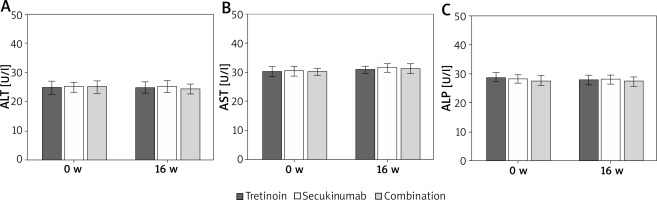
Contrast of liver enzyme indexes of subjects
As illustrated in Figure 4, serum ALT, AST, and ALP levels were similar before and after the intervention (p > 0.05).
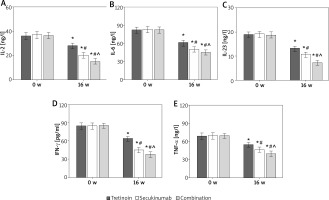
Contrast of IF indexes in subjects
After 16 weeks of intervention, the serum inflammatory factors (IFs) of subjects were notably lower compared to pre-therapy levels. The serum levels of IL-2, IL-6, IL-23, IFN-γ, and TNF-α in both the Secukinumab group and subjects receiving the combination therapy were significantly lower than those in the Tretinoin group. Furthermore, the levels of these inflammatory factors were significantly lower in subjects with the combination therapy compared to those in the Secukinumab group (all p < 0.05) (refer to Figure 5).
Assessment of treatment safety of subjects
After receiving medication, subjects treated with Tretinoin experienced 0 (0.0%) cases of the upper respiratory tract infection, 5 (11.1%) cases of pruritus, 1 (2.2%) case of erythema, resulting in a total adverse reaction rate of 13.3% (6/45). Subjects treated with Secukinumab reported 1 (2.2%) case of the upper respiratory tract infection, 2 (4.4%) cases of pruritus, and 0 (0.0%) case of erythema, leading to an overall adverse reaction rate of 6.7% (3/45). Subjects undergoing the combination therapy had 1 (2.2%) case of the upper respiratory tract infection, 1 (2.2%) case of pruritus, and 0 (0.0%) cases of erythema, resulting in an overall adverse reaction rate of 4.4% (2/45).
The adverse reaction rates for subjects treated with Secukinumab and those receiving the combination therapy were significantly lower compared to subjects treated with Tretinoin (p < 0.05). However, no significant difference was observed in the incidence of adverse reactions between subjects treated with Secukinumab and those undergoing the combination therapy (p > 0.05).
Discussion
Psoriasis is a systemic inflammatory disease. Sick persons mainly present with erythema and phosphorus dandruff. It can occur all over the body, but the limbs and scalp are more common. The pathogenesis of psoriasis is not clear, and its process is related to autoimmune dysfunction, hypertension, diabetes, dyslipidaemia, and metabolic syndrome [12–14]. There are massive IL-17 cells in the lesion area of psoriasis. Th17 cells produce the proinflammatory cytokine IL-17A, which can promote the proliferation of keratinocytes, inhibit the expression of a variety of cell adhesion factors, destroy the epidermal barrier structure, cause neutrophils to migrate and accumulate in skin lesions, and eventually cause inflammatory response [15, 16]. Secukinumab is the first fully human IL-17A inhibitor, which participates in the progression of psoriasis by blocking the expression of IL-17A. Studies have confirmed that secukinumab can rapidly reduce the clinical symptoms of sick persons with MSP and improve the quality of life of sick persons [17]. This article presents a comparative analysis for the clinical effect of Secukinumab plus Tretinoin therapy in MSP vulgaris. It was found that as against Tretinoin and Secukinumab alone, Secukinumab combined with Tretinoin therapy resulted in a clearly lower PASI score for sick persons.
T lymphocyte proliferation, Ifs, endocrine disorders, and other factors can also lead to the occurrence of psoriasis [18]. T lymphocytes can release a large number of cytokines through their own activation and trigger an inflammatory response. This molecular biochemical reaction will aggravate the symptoms of psoriasis. Tretinoin is a commonly used drug against abnormal skin keratinization, which can inhibit T cell proliferation and promote keratinocyte differentiation [19]. Tretinoin also has the inhibition of cutin cell proliferation and the role of leukocyte chemotaxis [20]. This article demonstrated that CD3+, CD4+, and CD4+/CD8+ in serum of sick persons increased markedly, while the proportion of CD8+ decreased markedly following the therapy with Tretinoin. Tretinoin could effectively improve the abnormality of T lymphocyte subsets in patients with MSP. Moreover, this article revealed that the combination therapy with Secukinumab and Tretinoin had markedly greater improvements in the proportion of CD3+, CD4+, CD8+, and CD4+/CD8+ in serum of sick persons than Tretinoin or Secukinumab alone. These results indicate that Secukinumab combined with Tretinoin can better improve the abnormality of T lymphocyte subsets in sick persons with MSP.
It was confirmed that psoriasis has a certain relationship with metabolic syndrome [21]. Most sick persons with psoriasis have abnormalities such as insulin resistance, diabetes, and hyperlipidaemia, which may be related to tissue damage and insulin resistance caused by chronic inflammation [22]. Metabolic syndrome can increase the incidence of psoriasis and increase mortality [23]. ApoA1 is an acute-phase negative regulatory protein, and its expression is down-regulated in the acute inflammatory phase [24]. ApoB is the major structural protein of LDL-C, which is responsible for transporting cholesterol and lipids to various tissues throughout the body [25]. It was found that the levels of TC, TG, LDL, HDL, FBG, ApoA1, and ApoB in patients with MSP vulgaris were not markedly changed, and the liver enzymes ALP, ALT, and AST also did not change markedly following the therapy. The results indicate that Secukinumab and Tretinoin have no effect on glucose and lipid metabolism and liver function in patients with psoriasis. The chronic inflammatory state caused by psoriasis can promote the release of purines from cells, thereby increasing UA to a certain extent [26]. In this article, Secukinumab combined with Tretinoin decreased blood UA levels in sick persons following the treatment. Secukinumab and Tretinoin combination can better improve UA metabolism in MSP sick persons.
Retinoic acid (RA) is present in multiple isoforms, with the most prevalent being all-trans retinoic acid and 9-cis retinoic acid [27]. All-trans-retinoic acid (ATRA) serves as a primary physiologically active metabolite of vitamin A. Due to their hydrophobic nature, retinoids necessitate retinoid-binding proteins for stability in aqueous environments. The stabilization of retinoids varies based on their location, achieved through binding to distinct proteins like cellular retinol-binding proteins (CRBPs) or cellular retinoic acid-binding proteins (CRABPs), as well as interstitial retinol-binding protein (RBP 3) and plasma-retinol binding protein (RBP 4) [28]. ATRA has demonstrated efficacy as a fungistatic agent, particularly against Candida and Aspergillus fumigatus. Consequently, it found application in the treatment of psoriasis in individuals prone to fungal infections [29]. Experimental findings indicated that ATRA induced a reduction in the Th17 population, an elevation in the CD4þ Treg population, and the stimulation of suppressive B cells [30]. ATRA’s potential in the treatment of psoriasis stems from its ability to regulate skin cell growth, differentiation, sebum synthesis, and collagen production through binding with retinoic acid receptors [31, 32]. A prior study proposed that ATRA could suppress the inflammatory response by significantly decreasing the expression levels of proinflammatory cytokines (e.g., IL-6, ICAM-1, and HLA-DR), presenting additional benefits for psoriatic treatment [33, 34].
Secukinumab distinguishes itself from other approved psoriasis therapies as a pioneering recombinant high-affinity, fully human monoclonal antibody belonging to the IgG1/kappa isotype. It is unique in its selective targeting of IL-17A, representing a first-in-class approach. Secukinumab disrupts the pathologic processes of psoriasis by specifically binding to IL-17A, preventing its interaction with the IL-17 receptor found on keratinocytes, thereby intervening in the disease’s progression. Through this mechanism, secukinumab not only prevents but also reverses key pathologic processes in psoriasis, ultimately leading to the normalization of skin histology [35].
At therapeutic concentrations used in psoriasis treatment, secukinumab fully neutralizes IL-17A’s activity without impacting IL-17F, preserving other functions of Th17 cells and avoiding direct influence on the Th1 pathway. This targeted action is a distinctive feature among IL-17 inhibitors, contributing to the normalization of skin histology and achieving clear to almost clear skin for the majority of patients. Importantly, this specificity holds the promise of fewer off-target effects when compared to other currently available treatment options [36].
This article found that the serum levels of IL-2, IL-6, IL-23, IFN-γ, and TNF-α were markedly lower in sick persons treated with Secukinumab and Tretinoin relative to sick persons treated with Tretinoin and Secukinumab alone. It revealed that Secukinumab plus Tretinoin therapy can better suppress the inflammatory response in patients with MSP.