Purpose
Prostate cancer is the second most prevalent cancer and the fourth leading cause of cancer-related mortality in men worldwide [1]. Various therapeutic options, such as surgery and radiotherapy (RT), exist for treating patients with localized prostate cancer [2, 3]. The choice of treatment is influenced by a range of factors, including clinical and pathological characteristics of the tumor, patient comorbidities, life expectancy, and individual preferences [4]. Toxicity profiles of these treatment modalities often serve as additional criteria for tailoring patient-specific approaches, especially when comorbidities are present [2, 3].
Inflammatory bowel disease (IBD) is a relative contraindication to external beam radiation therapy (EBRT) due to concerns that radiation dose to prostate-rectal interface and small and large bowel could precipitate significant acute and late rectal and bowel toxicity. One study found an overall incidence of severe toxicity of 46% following abdominal and pelvic irradiation among patients with IBD, with 21% experiencing acute enteral toxicity that necessitated cessation of RT, and late toxicity requiring hospitalization or laparotomy due to complications of the small or large bowel [5]. Existing literature on the safety of EBRT in IBD patients with prostate cancer shows an association between EBRT and increased risk of gastrointestinal (GI) toxicity, though perhaps somewhat mitigated with the use of a hydrogel rectal spacer [6, 7]. While advancements in radiation techniques have enabled more targeted delivery, EBRT still exposes the rectal-prostate interface and surrounding tissues in the lower pelvis to significant radiation. High-dose-rate brachytherapy (HDR-BT) is a precision-focused alternative that offers excellent local control while limiting dose to surrounding tissues [8, 9]. However, data on the safety of HDR-BT in patients with IBD is limited to small retrospective studies or institutional case series [10, 11].
This study aimed to evaluate the safety of HDR-BT, both as a monotherapy and as a boost to EBRT, in this specific patient cohort treated at the University of California San Francisco (UCSF).
Material and methods
This study was done in accordance with institutional ethical standards and the Declaration of Helsinki, and was approved by the Institutional Review Board at UCSF (IRB approval No.: 20-31257). A retrospective chart review was performed to identify patients diagnosed with Crohn’s disease (CD) or ulcerative colitis (UC), and treated for prostate cancer by two attending physicians at UCSF (OM and ICH) with HDR-BT between 2010 and 2022. Inclusion criteria were patients with biopsy-confirmed prostate cancer with no evidence of distant disease on clinical examination or imaging, no history of prior pelvic RT, and at least one post-HDR-BT treatment clinic appointment attended.
All patients received definitive HDR-BT at UCSF. Brachytherapy catheters were inserted through the perineum into the prostate using real-time trans-rectal ultrasound guidance. Sixteen catheters were implanted in all patients regardless of prostate cancer size, as previously described [12, 13]. Thereafter, patients underwent computed tomography (CT) of the pelvis for simulation and treatment planning. On CT images, the treating physician delineated the whole prostate clinical target volume (CTV) without margin and organs at risk (OARs), including the penile bulb, rectum, bladder, and intra-prostatic urethra. Seminal vesicles were included at the treating physician’s discretion. CTV to planning target volume (PTV) margin was 0 mm. Treatment planning was performed using inverse planning simulated annealing (IPSA) (Oncentra Brachy, Elekta, Sweden). Treatment planning goals were defined as dose-volume normalized to prescription dose. Target goal for prostate PTV was 100% of the prescription to 95% (preferred, 90% required) of the volume. OARs planning goals included a urethra constraint of no more than 1 cc receiving ≥ 120% of the prescription, and a maximum dose to 0.03 cc < 150% of the prescription. For the bladder and rectum or ileum in patients with prior colectomy, clinical goal was for < 1 cc receiving 75% of the prescription.
For this study, acute and late genitourinary (GU) and GI toxicities were retrospectively graded with common terminology criteria for adverse events (CTCAE), version 5. Toxicity was defined as acute if it occurred less than three months after completing radiation, and late toxicity was specified if it occurred more than three months later. Disease outcomes included post-treatment prostate specific antigen (PSA) and biochemical failure defined with Phoenix definition (nadir + 2 ng/ml) [14].
Results
Patients’ characteristics are described in Table 1. The median follow-up was 28.7 months (range, 4.2-95.9 months). Seven patients (63.6%) had UC, and one patient (9.1%) had active CD. Androgen deprivation therapy (ADT) was used in five patients (45.4%), with a median duration of 18 months (range, 7-24 months). The median baseline IPSS score was 8 (range, 3-26). SpaceOAR® rectal spacer was applied in seven patients, while the other patients were treated before rectal spacers were common.
Table 1
Baseline patients’ characteristics
[i] NCCN – National Comprehensive Cancer Network, LR – low-risk, UIR – unfavorable intermediate-risk, HR – high-risk, IBD – inflammatory bowel disease, UC – ulcerative colitis, CD – Crohn’s disease, ADT – androgen deprivation therapy, IPSS – international prostate symptom score, GU – genitourinary, GI – gastrointestinal, EBRT – external beam radiotherapy
In all patients, HDR-BT was used as definitive treatment. Nine patients were treated with HDR-BT without EBRT, including six patients with 2700 cGy in 2 fractions, and three patients with 3150 cGy in 3 fractions. Two patients (18.2%) were treated with a combination of EBRT to the pelvis to 45 Gy in 25 fractions and HDR-BT to 15 Gy in 1 fraction. The median V95% and V90% of the prescription were 96.4% and 98.3%, respectively. All patients achieved rectal and bladder constraints (Figure 1 and Table 2).
Fig. 1
Axial, sagittal, and coronal plane dose distributions from a single-fraction of high-dose-rate (HDR) brachytherapy used to treat the prostate in a patient with a concurrent inflammatory bowel disease diagnosis. The suture line from a prior surgery is evident in the sagittal image
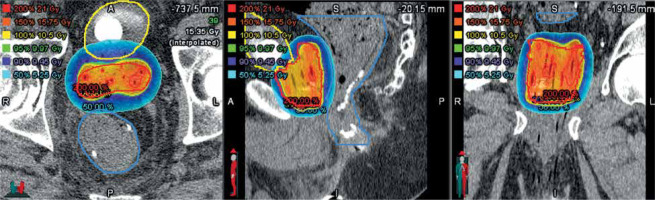
Table 2
Dosimetric parameters for patients treated with HDR brachytherapy
All patients experienced acute GU toxicity, with ten patients (91%) experiencing grade 1 toxicity and one patient (9%) grade 2 toxicity (Table 3). The most common acute GU toxicity was urinary frequency (82% grade 1 and 18% grade 2), with 45% reporting nocturia. Grade 1 incontinence and urgency were reported in one patient (9%). Eight patients (73%) had late grade 1 GU toxicity, and three (27%) had grade 2 GU toxicity. The most frequently reported late toxicity was urinary frequency (45% grade 1 and 27% grade 2), with 36% reporting nocturia. One patient (9%) had grade 2 erectile dysfunction. One patient (9%) had grade 1 hematuria that only occurred once. No grade 3 or higher late toxicity was observed in this cohort. One patient, who experienced intermittent diminished urinary stream, dysuria, and hematuria at 15 months after completion of HDR-BT, underwent a cystoscopy, which showed a proximal bulbar urethral stricture that prevented passage of a flexible cystoscope. Urinary cytology was negative for malignancy, and his urinary symptoms completely resolved without any intervention.
Table 3
Genitourinary toxicity grading after HDR brachytherapy
| Toxicity | Grade | n (%) |
|---|---|---|
| Acute GU toxicity | 1 | 10 (90.9) |
| 2 | 1 (9.1) | |
| 3 | 0 | |
| 4 | 0 | |
| 5 | 0 | |
| Late GU toxicity | 1 | 8 (72.7) |
| 2 | 3 (27.3) | |
| 3 | 0 | |
| 4 | 0 | |
| 5 | 0 |
Six patients (54.5%) had grade 1 acute GI toxicity (Table 4). No grade 2 or higher acute GI toxicity was observed. Six patients (54.5%) experienced late grade 1 GI toxicity, and one patient (9.1%) had late grade 2 GI toxicity. Four patients experienced acute and late grade 1 diarrhea (36%). Two patients (18%) had acute rectal incontinence, one patient (9%) developed late rectal leakage, and one patient (9%) experienced low-grade dysplasia.
Table 4
Gastrointestinal toxicity after HDR brachytherapy
| Toxicity | Grade | n (%) |
|---|---|---|
| Acute GI toxicity | 1 | 6 (54.5) |
| 2 | 0 | |
| 3 | 0 | |
| 4 | 0 | |
| 5 | 0 | |
| Late GI toxicity | 1 | 6 (54.5) |
| 2 | 1 (9.1) | |
| 3 | 0 | |
| 4 | 0 | |
| 5 | 0 |
All patients achieved a PSA response to treatment. The six patients, who were treated without ADT had a median PSA of 6.6 ng/ml (range, 4.5-11.2 ng/ml) at diagnosis. At a median of 12 months after ADT initiation, these patients achieved a median PSA of 1.5 ng/ml (range, 0.4-3.4 ng/ml). No patient experienced biochemical or clinical recurrence, distant metastasis, or death from any cause.
Discussion
The current study investigated the safety, effectiveness, and tolerability of HDR-BT in patients with localized prostate cancer and IBD. Our cohort achieved notable PSA responses in all patients, and none experienced severe GI or GU toxicities during a median follow-up of 28.7 months. This study adds to the literature supporting the safety of HDR brachytherapy in patients with IBD and localized prostate cancer.
Previous brachytherapy studies on patients with prostate cancer and IBD have primarily focused on the safety of low-dose-rate brachytherapy (LDR-BT), and have reported conflicting toxicity results [15, 16]. Grann et al. performed a retrospective analysis of six patients with prostate cancer and IBD treated with iodine-125 (125I) LDR-BT, and reported no severe GI toxicity [17]. In a larger cohort of 24 patients treated with EBRT and LDR-BT, 17% of patients had grade 2 rectal toxicity, and no acute or late grade 3 or 4 rectal toxicity was reported [15]. In contrast, Pai et al. conducted a review of 13 patients treated with 125I LDR-BT monotherapy, and reported grade 3 or higher acute and late GI toxicity in 23% and 15% of patients, respectively [18]. All patients in this study, who experienced severe GI toxicity had completed an endoscopic assessment or biopsy of the rectum related to IBD within 3 months of brachytherapy [18]. Avoidance of biopsies or endoscopic assessment within the first few months after brachytherapy may help reduce the risk of late GI toxicity related to instrumentation of recently irradiated tissue. In a recent systematic review on radiotherapy in 194 patients with IBD treated with various modalities, including LDR-BT, HDR-BT, EBRT, and stereotactic body radiotherapy, late grade 3 or higher GI toxicity rate was 2.3% [19]. Similarly, a report on patients with prostate cancer and IBD treated with radiation (two with brachytherapy monotherapy, and eight with EBRT and brachytherapy boost) showed that IBD located in the rectum and low body mass index were associated with more severe rectal toxicity within six months of radiation [20]. These data suggest that patients with rectal or recto-sigmoid involvement of IBD may be at higher risk of severe GI toxicity. However, Peters et al.’s cohort included 14 patients with rectal or recto-sigmoid involvement, and found that LDR-BT was well-tolerated without severe toxicity [15].
When compared with EBRT, brachytherapy has been associated with similar or improved GI toxicity in patients with prostate cancer and IBD. In a retrospective cohort study of 100 patients with IBD and prostate cancerRT was associated with a two-fold increase in the rate of IBD flare, and there was no statistically significant difference in the rates of IBD flare when comparing EBRT vs. brachytherapy at 6 months (11.8% vs. 7.7%, p = 1) [21]. Tromp et al. conducted a systematic review on bowel toxicity in patients with IBD treated for cancer with RT. Eight studies were included, with three studies assessing the use of brachytherapy, in which 7% of patients received brachytherapy [22]. The authors found that brachytherapy had similar rates of toxicity in patients with and without IBD, while EBRT was associated with increased rates of acute and late toxicity. The incidence of grade 3 or higher acute bowel toxicity was 7% in brachytherapy studies compared with 20% in EBRT studies, with similar numbers for late bowel toxicity (5% for brachytherapy vs. 15% for EBRT). In our cohort, only two patients received EBRT, and no grade 3 or higher GU or GI toxicity was identified.
There is limited data suggesting that HDR-BT has a more favorable toxicity profile compared with LDR-BT. Mohammed et al. described the safety and tolerability of HDR-BT in a small retrospective cohort of 11 patients with prostate cancer and IBD, and reported grade 1 diarrhea in three patients, grade 1 proctitis in three patients, and no grade 2 or greater rectal toxicity [10]. They concluded that HDR-BT has a better toxicity profile relative to LDR-BT due to the inverse planning advantage of HDR-BT. Lehrich et al. reported on patients with prostate cancer and IBD, with 70% receiving a hydrogel rectal spacer at the time of EBRT with HDR-BT boost [7, 23]. No patient experienced severe acute or late proctitis or diarrhea [11]. In our cohort, seven patients received rectal spacers, and no severe GI toxicity was observed. The use of rectal spacers is encouraged in this patient population if feasible for protection of the anorectum.
Our study, however, has its limitations as a retrospective study with a small cohort, which is susceptible to selection bias and limited generalizability. The follow-up duration of 28.7 months is relatively short. Moreover, our cohort lacks patients with active IBD and rectal involvement, known to be potential predictors of GI toxicity. Fecal calprotectin level was not available for this study, which can be a helpful surrogate marker of GI inflammation to help distinguish between IBD activity and radiation-related GI toxicity, given the high negative predictive value of calprotectin [24, 25]. Due to the cohort’s small size, our study did not have sufficient statistical power to identify clinical or dosimetric predictors of GI toxicity or IBD flare-ups. Despite these limitations, our results show that HDR delivered with limited rectal dose is safe and well-tolerated in patients with prostate cancer and IBD, with a low-risk of IBD flare, GI toxicity, or GU toxicity.


