Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by pruritus, xerosis, recurrent and/or persistent erythematous papules and plaques among other clinical features [1]. AD is predominantly mediated by a type 2 immune response with increased production of proinflammatory cytokines, particularly interleukin (IL) 4 (IL-4) and IL-13 [2]. These cytokines are mediated by the JAK-STAT signaling pathway with Janus kinase 1 being the key intracellular protein that mediates cytokine receptor signaling for IL-4 and IL-13 [2].
Mild AD is generally treated with topical treatments [3, 4]. However, patients with moderate to severe AD often require systemic and/or advanced therapies including biologic agents (dupilumab and tralokinumab) or selective Janus kinase-1 (JAK-1) inhibitors such as abrocitinib and upadacitinib [5].
JAK inhibitors are small molecules that target four intracellular proteins (JAK1, JAK2, JAK3, and tyrosine kinase 2), thereby interfering with the JAK-STAT pathway in lymphocytes. The JAK-STAT pathway regulates the activation and differentiation of T and B lymphocytes and the monocyte/macrophage compartment. This is relevant for both innate and adaptive immune responses against different pathogens; in the context of herpes zoster (HZ), advanced AD therapies target pathways associated with the immunity. The inhibition of the JAK-STAT pathway has been implicated in an increased risk of HZ, with this relationship being demonstrated in upadacitinib treatment of rheumatoid arthritis [6, 7]. Additionally, HZ has been associated with a statistically significant increase in IL-4, indicating that IL-4 may play a role in protecting against HZ [8]. As such, IL-4 inhibition may also be associated with an increased risk of HZ.
Aim
We performed a systematic review and network meta-analysis of randomized controlled trials (RCTs) to evaluate and compare the incidence and risk of HZ among patients with moderate to severe AD treated with advanced systemic therapies.
Material and methods
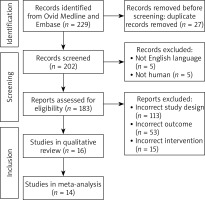
This systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1) [9]. The protocol was registered in the PROSPERO international prospective register of systematic reviews (CRD42022355842).
Search strategy
Systematic searches were conducted in Ovid MEDLINE and Embase from inception to December 10, 2022. The last search was performed on December 16, 2022. The full electronic search strategy used for each database is available in the supplement (Supplementary 1).
Eligibility criteria
We included RCTs, open label clinical trials and prospective cohort studies that included patients with AD who were given dupilumab, tralokinumab, abrocitinib, or upadacitinib. We did not include any time restrictions. Sensitive eligibility criteria were used for the title and abstract screening. All studies that reported the incidence of HZ were selected for full text screening. A study was included in the meta-analysis if it reported 1) quantitative data on the incidence of HZ in patients with AD and 2) had both a placebo/control group and an intervention group that included at least one of the eligible advanced therapies (dupilumab, tralokinumab, abrocitinib, or upadacitinib).
Screening
Two reviewers (N.N. and D.M.) independently assessed all titles and abstracts for relevance according to the sensitive eligibility criteria. When duplicates were identified, the most recent study was included. Full-text screening was performed by the same two reviewers (N.N. and D.M.) in accordance with established eligibility criteria. Disagreements during screening were resolved by a third reviewer (E.S.).
Data extraction
Two reviewers (N.N. and D.M.) independently extracted the relevant data from the eligible studies into a standardized Microsoft Excel file. Disagreements were resolved following discussion, and the final decision was reached via consensus with the third reviewer (E.S.). The extracted data included study design and characteristics (first author, date of publication), number, sex, age, incidence rate of HZ in control and intervention groups and follow-up period (Supplementary 2).
Primary outcome
The primary outcome was the incidence of HZ in: 1) control/placebo with AD or 2) patients with AD receiving at least one of the following agents: dupilumab, tralokinumab, abrocitinib, or upadacitinib.
Risk of bias assessment
Risk of bias was assessed independently by two reviewers (D.M. and E.S.) using the Cochrane Risk of Bias 2 Tool [10]. The following five domains were evaluated for having low, some concerns or high risk of bias: risk of bias arising from the randomization process, risk of bias due to deviations from the intended interventions, missing outcome data, risk of bias in measurement of the outcome, risk of bias in the selection of the reported result (Supplementary 3).
Data synthesis
We conducted all analyses in Stata (StataCorp., Version 17) [11]. For our primary outcome of interest, we expressed our effect estimates as odds ratios (ORs) with corresponding 95% confidence intervals (CIs), to assess which interventions were associated with a lower incidence of herpes. Our reference comparator was the placebo intervention.
We conducted random-effects, frequentist NMAs, i.e., multivariate meta-analysis, for the outcome, incidence of HZ, assuming a common heterogeneity parameter [12, 13] with the mvmeta command [14, 15] and network suite 6 in Stata. We first used the global test of the “design-by-treatment” model to check the coherence assumption in our entire network [16]. We then ran the node-splitting model to assess for incoherence between direct and indirect effect estimates [8, 9]. If incoherence was observed between direct and indirect effect estimates, we used the IF plot to assess loop-specific incoherence [17]. Ranking probabilities for the interventions in our two networks were estimated using the surface under the cumulative ranking curve (SUCRA) values, mean ranks and rankograms [14, 17].
For the purposes of this analysis, different dosages of certain advanced systemic therapies were classified as unique interventions to elucidate any possible dose-dependent effects. Additionally, we combined arms for multi-arm trials which examined the same intervention, by combining the number of patients and sample sizes across the respective arms. Studies with 0 patients in both arms were automatically excluded from the analysis by the statistical software program. Lastly, the transitivity assumption was completed based on the quantity of trials included in the review.
Results
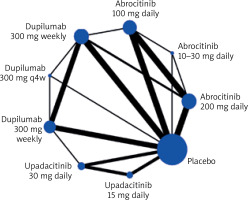
The literature search yielded 183 unique records. After screening titles and abstracts, 35 articles were retrieved for full-text evaluation; 16 studies satisfied the predetermined eligibility criteria and 14 were included in this meta-analysis as shown in the PRISMA flow diagram (Figure 1). After eligibility criteria were applied, our final search yielded no studies that included tralokinumab. A network map was created to show the associations and their strengths between all groups for the network meta-analysis (Figure 2).
Figure 2
Network map for the risk of HZ by various interventions; both direct and indirect comparisons. The node size (circle size) reflects the number of patients allocated to the group, whereas the connection size (line thickness) is in proportion to each direct comparison
Study characteristics
The studies included a combined total of 10,689 patients with a mean age of 35 years and 58.7% male. The mean follow-up period of the studies was 30.2 weeks. Additional details and baseline characteristics are presented in Supplementary 2.
Quality assessment
Based on the Cochrane Risk of Bias Tool [10], the methodological quality of most of the studies was high with low risk of bias with some papers demonstrating some concerns regarding bias. A final judgment for each paper was then made for overall risk of bias. Discrepancies were resolved via reviewing studies in consensus. Two RCT papers demonstrated a high risk of bias, with one paper only providing interim results for the clinical trial. Both prospective cohort studies included in this paper were considered high risk of bias, but these papers were only included in the systematic review and not the network meta-analysis. Details of the quality assessments can be found in Supplementary 3.
Incidence of herpes zoster
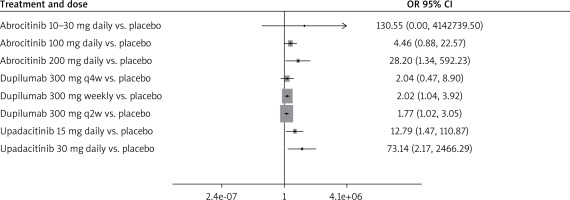
When compared to placebo, all treatment arms except dupilumab 300 mg every 4 weeks, and abrocitinib 100 mg once daily demonstrated a statistically significant association with an increased risk of HZ, according to the forest plot (Figure 3). However, it should be noted that although dupilumab 300 mg weekly and dupilumab 300 mg every 2 weeks demonstrated a statistically significant association, the ORs were close to the no-effect line (2.02 [1.04, 3.92] and 1.77 [1.02, 3.05], respectively).
JAK-1 inhibitors
Upadacitinib at 15 mg and 30 mg doses were associated with an increased risk of HZ when compared to placebo, and when compared to dupilumab weekly and every 2 weeks (Table 1). Our results also showed a dose-dependent effect with upadacitinib. While both the 15 mg and 30 mg doses were associated with a significant increase in HZ compared to placebo; upadacitinib 30 mg resulted in a higher incidence of HZ compared to the 15 mg dose (Table 1).
Table 1
League table
[i] League table showing the comparative risk of HZ included for different interventions in this network meta-analysis. Upadacitinib 15 mg and 30 mg caused a significant increase in HZ when compared to various frequencies of dupilumab and placebo. Abrocitinib 200 mg caused a significant increase in HZ when compared to placebo. Bold text represents statistically significant comparisons, while non-bold boxes indicate non-statistically significant comparisons.
Abrocitinib 200 mg once daily also resulted in a statistically significant increase in HZ when compared to placebo (Figure 3, Table 1). Additionally, abrocitinib 100 mg and 200 mg illustrate a potential dose-dependent effect where abrocitinib 200 mg resulted in a higher incidence of HZ than abrocitinib 100 mg (Figure 3, Table 2).
Table 2
SUCRA table
[i] Surface Under the Cumulative Ranking Curve (SUCRA) ranks showing the probability of each treatment group causes the least incidence of HZ (highest to lowest). Abrocitinib 100 mg with SUCRA 53.1, versus abrocitinib 200 mg with SUCRA 22.2 implies a dose-dependent effect. Upadacitinib 15 mg with SUCRA 34.5 versus upadacitinib 30 mg with SUCRA 12.7 show a similar dose-dependent effect.
IL-4/13 inhibitors
Dupilumab administered weekly and every 2 weeks had a statistically significant increase in HZ incidence when compared to placebo. However, dupilumab every 4 weeks crossed the no-effect line, losing statistical significance for assessing HZ incidence (Figure 3).
Overall, there is a clear demarcation between IL-4/13 inhibitor (dupilumab) and the JAK-1 inhibitors (abrocitinib and upadacitinib) that were evaluated by this study.
Discussion
We report a network meta-analysis examining the risk of HZ in patients with AD being treated with either dupilumab, upadacitinib or abrocitinib including data from over 10,000 patients in 14 separate trials. The results demonstrate a statistically significant increase of HZ with JAK-1 inhibitors when compared to placebo or dupilumab administered weekly or every 2 weeks.
HZ is associated with acute and long-term symptoms including post-herpetic neuralgia and potentially rare life-threatening complications such as encephalitis [18]. Due to the immunomodulatory effects of IL-4/13 inhibitors and JAK-1 inhibitors, the immune response against the varicella zoster virus may be impaired in patients receiving these treatments. Patients with AD are more prone to HZ and therefore, investigating the risk of developing HZ in AD patients treated with either IL-4/13 or JAK-1 inhibitors will further elucidate the safety of these treatments in this population and may further inform clinical practice and decision making when considering treatment options for AD.
Overall, according to SUCRA rankings, upadacitinib 30 mg once daily, abrocitinib 200 mg once daily, and upadacitinib 15 mg once daily are the most, second most, and third most likely drugs to increase the risk of HZ in patients with AD (Table 2). These differences were statistically significant compared to placebo. Contrarily, dupilumab is the least likely to cause HZ compared to all other treatments and placebo.
Upadacitinib was shown to have a dose-dependent effect in increasing incidence of HZ. Upadacitinib 30 mg is associated with a higher risk of HZ (mean SUCRA rank of 8) compared to upadacitinib 15 mg (mean SUCRA rank of 6.2) (Table 2). Upadacitinib has been shown to increase the risk of HZ in inflammatory bowel disease and rheumatoid arthritis [7, 19]. Our review is the first NMA to demonstrate a statistically significant dose-dependent increase in HZ in patients with AD receiving upadacitinib.
A dose-dependent increased incidence of HZ was also observed with abrocitinib. Abrocitinib 200 mg is associated with a higher risk of HZ (mean SUCRA rank of 7.2) compared to abrocitinib 100 mg (mean SUCRA rank of 4.7) (Table 2). Abrocitinib at very low doses of 10 mg or 30 mg daily (ABLOW) seemed to have results inconsistent with the rest of the data. However, since these dosages are not used clinically, the sample size for this group was incredibly small, creating large confidence intervals and inconclusive results. These doses were prepared specifically for the early phase 2 trials as the typical abrocitinib doses in clinical practice are 100 mg or 200 mg once daily.
While dupilumab 300 mg weekly and dupilumab 300 mg every two weeks demonstrated a statistically increased risk of HZ compared to placebo, the ORs were close to no-effect. Dupilumab every 4 weeks had no statistically significant difference in HZ when compared to placebo. Although it might be expected for the placebo to have the lowest rate of HZ, the SUCRA ranks showed the dupilumab intervention groups having higher probabilities of curtailing the incidence of HZ when compared to placebo. A possible reason for these unanticipated results could be that targeted treatment with dupilumab ameliorates the epidermal barrier dysfunction in AD.
Antiviral innate defenses are associated with type 1 interferons (e.g. IFN-α and IFN-β) and type 2 interferons (e.g. IFN-γ) [20]. These interferons rely upon the JAK-1 signaling; the adaptive response to viruses is predominantly related to Th1 cytokines (e.g. IFN-γ) whose intracellular pathway is mediated by JAK 1 and 2 [21]. Additionally, antiviral CD8+ T cells require activation of JAK pathways [22]. Therefore, JAK pathway inhibition could be associated with an increased susceptibility to viral infections, dissemination and reactivation.
Our research shows that JAK-1 inhibitors have a statistically significant increase in HZ in AD patients, and considering the complications associated with HZ, such as post-herpetic neuralgia, we believe that clinicians should consider this when deciding between prescribing IL-4/13 inhibitors and JAK-1 inhibitors. This is especially true for those at increased risk of HZ such as patients above 50 years of age.
The recombinant HZ vaccine has been shown to be safe and effective in reducing the incidence and severity of HZ and post-herpetic neuralgia [23, 24]. Considering the significant increase of HZ incidence in those using JAK-1 inhibitors, our research confirms the ongoing clinical consensus to use the recombinant zoster vaccine for AD patients undergoing treatment with JAK-1 inhibitors. This would also alleviate the burden on patient quality of life and optimizes the use of healthcare resources as the HZ vaccine is a relatively cheaper, low-risk and low-patient burden intervention.
This network meta-analysis has several limitations. Firstly, the small sample size of our study was restrictive as it prevented the assessment of the transitivity assumption and the examination of the substantial heterogeneity observed through sub-group analyses and meta-regression. This limitation, coupled with the incoherence observed in some loops highlights a limited power in our analysis at addressing the comparative effectiveness of included interventions. Another possible limitation is the inclusion of 2 trials that had a high risk of bias; however, as these results are consistent with all the other studies, the quality of those two trials is unlikely to influence the overall robustness of results. Another limitation of our NMA is the over-representation of dupilumab as there were more studies evaluating dupilumab than JAK-1 inhibitors. The earlier approval of dupilumab for AD treatment could contribute to the paucity of literature for JAK-1 inhibitors, ultimately resulting in a more robust dataset for dupilumab. This over-representation could be mitigated by further research of JAK-1 inhibitors and other biologics like IL-13 inhibitors (e.g. tralokinumab or lebrikizumab) for treatment of AD.
Conclusions
Usage of JAK-1 selective inhibitors such as abrocitinib and upadacitinib have a significantly increased risk of HZ when compared to dupilumab. A dose-dependent response was demonstrated with the JAK-1 inhibitors. Clinicians should consider these findings when considering advanced therapies for patients with moderate to severe AD. Recombinant vaccines against HZ should be strongly considered prior to initiating JAK-1 inhibitors.