Introduction
Worldwide cervical cancer (CC) is one of the most frequently diagnosed oncological diseases among women and a major cause of morbidity and mortality. 600,000 new cases were diagnosed in 2020, and more than 300,000 ended in patient death, according to GLOBOCAN [1]. There are 2 main ways of CC prevention: through the human papilloma virus (HPV) vaccine and with a population-wide screening program containing a Papanicolaou test and HPV screening. However, the incidence rate of this disease keeps on rising in developing countries due to a lack of screening and a low vaccination rate – around 90% of new cases and deaths from CC are happening in low- and middle-income countries [2]. There are several risk factors described in the literature that can lead to CC: poor economic status, multiple sexual partners, low sexual hygiene, smoking, and infection with HPV [3].
Cervical cancer is most frequently associated with infection with high-risk HPV viral strains 16 and 18, which are responsible for 58% and 16% of all CC cases in Europe, respectively [4]. However, CC is a multifactorial disease; less than 10% of patients infected with HPV develop carcinoma in situ [5], which, if left untreated, progresses to invasive cancer. In addition to the viral infection, other lifestyle and epigenetic factors such as smoking, chronic inflammation, and co-infection with other sexually transmitted diseases can increase the risk for CC development in HPV-infected patients [6].
Several of the above listed factors can be associated with DNA damage, oxidative stress, and formation of reactive oxygen species (ROS), which lead to cancer initiation and progression [6]. High-risk HPV strains 16 and 18 encode 2 major viral oncogenes: E6 and E7 [7]. The E6 oncoprotein expression increases the amount of ROS in HPV-infected cells [7]. Reactive oxygen species such as the hydroxyl radical, hydrogen peroxide, and the superoxide anion radical are produced in all respiring cells under aerobic conditions, the main site of their production being the mitochondria. Under normal physiological conditions there is a balance between the production and elimination of ROS by various antioxidants and enzyme complexes. However, when the cellular conditions are at an imbalance, the production of ROS prevails over their elimination, a process called oxidative stress. Under oxidative stress, the increased production of ROS triggers a free radical chain reaction. This in turn leads to oxidation of polyunsaturated fatty acids (PUFAs) in cell membranes and the formation of reactive lipid electrophiles [8]. One of those electrophiles and the most extensively studied and biologically active alkenal is 4-hydroxy-2-nonenal (4HNE) [8].
Oxidative stress and ROS are widely recognised to be produced during cancer initiation and progression [9].
Mitochondria are the main site for production of ROS and 4-hydroxynonenal (4-HNE) [10, 11]. Furthermore, evidence suggests that mitochondrial macromolecular derivatives from 4-HNE may play a role in cancer initiation and progression [8]. Because we know that 4-HNE is a product of ROS, we aimed to evaluate its expression in different stages of CC as a possible prognostic factor.
Material and methods
Ethical Committee approval was obtained (number 656/29.06.2021) to investigate retrospectively the paraffin-embedded tumour tissue from 69 women with squamous CC.
Patients were diagnosed between 2015–2020 at our Department of Pathology; the studied cases were randomly selected (until the predetermined number was dialed – 69) from archival lists of the Department of Pathology. Information about the clinicopathological characteristics was obtained from hospital medical records.
Patients’ characteristics
We classified the patients using International Federation of Gynaecology and Obstetrics (FIGO) (2018) and the eighth edition of tumour-node-metastasis classifications. The distribution of patient’s according to both staging classifications is shown in Table 1. FIGO stage III includes only patients with lymph node metastases – FIGO IIIC. According to the current guidelines for treatment of CC for patients in stage FIGO IIIA and FIGO IIIB, primary surgery is not recommended.
Immunohistochemical scores
Currently, there is no accepted scoring system for 4-HNE. We implemented some of those used in the reporting of other markers. For each patient, we selected one slide with haematoxylin-eosin staining. From the corresponding formalin-fixed and paraffin-embedded tumour block, we stained one section with 3 µm thickness for 4-HNE (Clone HNEJ-2, Mo, dilution 1 : 150, Abcam, UK) and for ROMO1 (Clone OTI2C12, Mo, dilution 1 : 150, Abcam, UK). We used immunohistochemistry with visualisation EnVision ™ FLEX, High pH (DAKO) system, and AutostainerLink 48 technique (DAKO). We performed heat-mediated antigen retrieval with citrate buffer pH 6 before commencing with the immunohistochemistry staining protocol. As a positive external control, we used a colon adenocarcinoma tissue included in each run. The entire tissue section was evaluated at low and after that at high magnification by considering 2 indicators:
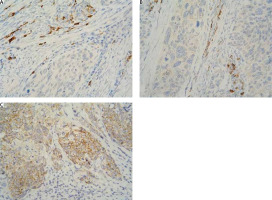
The localisation of expression in tumour cells (cytoplasmic/membrane/nuclear) was reported. When determining positivity, only membrane staining was included (Fig. 1). The result was interpreted based on 2 reporting models: H-score and Allred score. The 2 systems classify carcinomas into similar, but not identical, groups.
Fig. 1
Immunohistochemistry staining with 4-hydroxynonenal in cervical squamous cell carcinoma: cytoplasmic positivity absent (A), weak (B), and moderate (C) intensity in tumour cells; strong intensity in immune cells (A, B 400×)
H-score
For H-score assessment, the following formula was applied:
4-HNE H-score = (% of cells stained at weak intensity 1×) + (% of cells stained at moderate intensity 2×) + (% of cells stained at strong intensity 3×).
The resulting scores ranged 0–300, where 300 was equal to 100% of tumour cells stained strongly (3+).
Expression level was categorised according to the median value of the H-score (with H-score ≤ 15.79) or high (with H-score > 15.79). Greater than 1% positive cells with H-score = 0 was considered to be a negative result.
Allred score
For the Allred score, the following formula was used:
proportion score (PS) – 0 (no cells staining); 1 (< 1% cells staining); 2 (1–10% cells staining); 3 (11–33% cells staining); 4 (34–66% cells staining); 5 (67–100% cells staining); intensity score (IS) – 0 (no staining); 1 (weak staining); 2 (moderate staining); 3 (strong staining).
Total score (TS) = PS + IS, TS range = 0, 2–8
TS 0 and 2 were considered negative. Scores of 3–8 were considered positive (Table 2).
Table 2
Allred score
| Intensity score | Proportion score (% stained cells) |
|---|---|
| 0 (no staining) | 0 No cells |
| 1 (weak staining) | 1 < 1 |
| 2 (moderate staining) | 2 1–10 |
| 3 (strong staining) | 3 11–33 |
| 4 34–66 | |
| 5 67–100 |
Table 3
Distribution of patients according to International Federation of Gynaecology and Obstetrics stage and the final immunohistochemical scores
Combined score
For the combined score, we reported 2 indicators:
degree of intensity: missing (0 pts), weak (1 pts), moderate (2 pts), strong (3 pts),
percentage of positive tumour cells: no positive cells (0pts), 1–5% (1 pts), 6–25% (2 pts), 26–50% (3 pts), 51–75% (4 pts), 76–100% (5 pts).
The result was obtained based on the summation of the points from the 2 categories: negative result, with complete/nearly complete lack of expression (0–2 pts); weak expression (3–6 points); overexpression (7–8 points).
Statistical methods
Distribution of patients per group was summarised using standard descriptive measures such as counts and percentages (Table 1). Comparisons for 4-HNE H-score between more than 2 groups were performed using non-parametric Kruskal-Wallis test. Two-group comparisons for 4-HNE H-score were performed using two-sided Wilcoxon rank-sum test. P-values below 0.05 were considered statistically significant. All tests were implemented using R statistical environment for Windows (version 4.2.0). All plots were generated using R packages ggpubr (v. 0.4.0) and ggplot2 (v. 3.3.5). No formal sample size estimation was performed because of a lack of any reliable data for expression of 4-HNE in CC.
Results
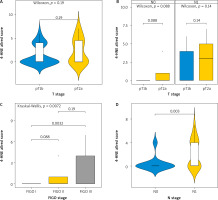
Distribution of patients according to FIGO stage and the final immunohistochemical scores. Using several techniques, we assessed 4-hydroxynonenal expression in cervical cancer patients at various tumour stages.
H-score
When we compared the expression of 4-HNE levels in different T stages (T1b and T2A), we observed no statistically significant difference, depending on the infiltration of the tumour in neighbouring structures (p-value = 0.2) (Fig. 2 A). There was no statistically significant difference in the expression of 4-HNE when comparing different tumour stages (T1b vs. T2A) in patients with and without metastatic lymph nodes (N0 vs. N1), with p-values of 0.089 and 0.14, respectively (Fig. 2 B). However, when comparing the expression of 4-HNE using the FIGO classification, we found a statistically significant difference between tumours in FIGO stage 1 and FIGO stage 3 (p = 0.0033) (Fig. 2 C).
Fig. 2
Kruskal-Wallis test for association between tumour-node-metastasis T stage and H-score (A), T stage and H-score in node-negative and node-positive patients (B) FIGO stage and H-score (C), N stage and H-score (D)
FIGO – International Federation of Gynaecology and Obstetrics
Lymph node involvement in the process also altered the expression of the marker (p = 0.0031), independently of the tumour size (D).
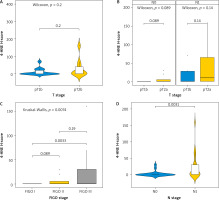
Allred score
When we used the Allred score to compare the expression of 4-HNE we obtained similar results (Fig. 3). The levels of expression of 4-HNE in different T stages (T1b and T2A) was not significantly different and did not depend on the infiltration of the tumour into neighbouring structures (p-value = 0.19) (Fig. 3 A). Comparing the N status at different T stages (Fig. 3 B), there was still no difference in expression of 4HNE (p = 0.088 and p = 0.14, respectively). Comparing the expression of 4-HNE in tumours between FIGO stage 1 and FIGO stage 3 (Fig. 3 C), there was a statistically significant difference (p = 0.0032). No difference in expression was seen between tumours in FIGO stage 1 and 2 or between FIGO stage 2 and 3. The expression of 4-HNE was higher in tumours where positive lymph nodes were also found (p = 0.003).
Combined score
The combined score for expression of 4-HNE showed similar results (Fig. 4). No statistically significant difference was observed between tumours with different sizes (T1b vs. T2A, p = 0.2) (Fig. 4 A). There was also no difference according to the involvement of lymph nodes by the process (p = 0.089 and p = 0.15, respectively).
Fig. 4
Kruskal-Wallis test for association between tumour-node-metastasis T stage and combined score (A), T stage and combined-score in node negative and node positive patients (B), FIGO stage and combined score (C), N stage and combined score (D)
FIGO – International Federation of Gynaecology and Obstetrics
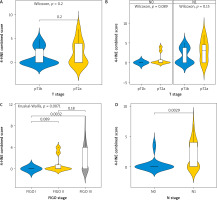
A significant difference was found comparing the expression of 4-HNE in tumours in FIGO stage 1 and FIGO stage 3 (p = 0.0032) (Fig. 4 C).
The expression of 4-HNE was higher again in tumours with already involved lymph nodes N1 (p = 0.0029).
Discussion
Oxidative stress and the increased production of ROS are known to be closely related to cancer initiation and development, and CC is no exception [12]. Our results show that the levels of 4-HNE are significantly higher in the more advanced stages of CC, compared to the early stages of the disease and in tumours that already have the ability to metastasise in lymph nodes, measured with all 3 immunohistochemical scores (the H-score, the Allred score, and the combined score). The results were similar regardless of which immunohistochemical method was used.
Bearing in mind that the histological appearance of 4-HNE depends on the histological origin of the cancer and that CC is a virally induced disease, we asked ourselves the question if and how HPV targets the cell to reprogram its metabolism in terms of protein and lipid synthesis, leading to higher 4-HNE expression in advanced staged of CC.
There are 3 cooperating processes between HPV and ROS that lead to carcinogenesis: genotoxicity, genomic instability caused by the virus, and the direct action of the E6 protein on carcinogenic HPV subtypes that increases ROS [13]. Lipid peroxidation, brought on by oxidative stress, is linked to tumour growth and development [14]. One of the most extensively studied biomarkers for oxidative stress and a product of lipid peroxidation is 4-HNE. It acts as a “second messenger” for free radicals, thus taking part in multiple signalling pathways [14]. Specifically, whereas ROS have a short half-life and can travel only a minimal distance, 4-HNE has a high affinity for binding to proteins, DNA, and phospholipids, resulting in the formation of very durable adducts. Therefore, 4-HNE can move freely from one place to another and alter the structure and function of proteins and DNA. Consequently, 4-HNE can alter the proliferation, differentiation, and death of cancer cells, as well as the functionality of the genome. Due to its potential to covalently bond to DNA, 4-HNE is regarded as a significant element in carcinogenesis; nevertheless, it may also be cytotoxic to cancer cells and control their growth [15]. By forming these strong covalent bonds with molecules containing an amino group (DNA, proteins phospholipids), 4-HNE acts as a growth regulating signalling factor [14].
The main source of ROS in eukaryotic and cancer cells are the mitochondria, by generating adenosine triphosphate (ATP) through oxidative phosphorylation. Vast quantities of superoxide are released during this process in complexes I and III of the electron transport chain. The majority of superoxide is converted by superoxide dismutase to hydrogen peroxide, another member of the ROS family. Reactive oxygen species target the membrane lipid bilayers, specifically the PUFA chains in lipids, as one of the main targets. Peroxidation of PUFAs creates a variety of primary lipid oxidation products and lipid electrophiles, 4-HNE being one of the best-studied active lipid electrophiles [16]. In contrast to its well- established biological effects, the chemical pathways that lead to the creation of 4-HNE remain obscure [17]. It is widely believed that 4-HNE originates from the decomposition of hydroperoxide of ω-6 PUFAs [8]. Consequently, phospholipids containing linoleic acid and arachidonic acid on cytoplasmic membranes are regarded as the primary source of 4-HNE synthesis. However, recent evidence shows that 4-HNE can be produced through oxidation of cardiolipin – a major mitochondrial phospholipid, rich in linoleic acid chains [8]. Reactive oxygen species target these chains, resulting in 4-HNE production. This process is also involved in cancer. Studies show that, compared to neighbouring tissue, human kidney cancer tissue shows increased immunohistochemical staining for 4-HNE protein metabolites in the cytoplasm and mitochondria [18]. Furthermore, there is evidence that the formation of 4-HNE protein metabolites in renal and colon cancer tissues has been linked to their growth and progression [19]. Increased 4-HNE levels have been linked to the development of liver cancer in animal models and humans [20]. Biasi et al. [21] showed that the levels of 4-HNE decrease in colon carcinoma tissues, compared to healthy ones, the only exception being tumours at the most advanced stage. In brain tumours, studies show that the levels of 4-HNE increase with the stage of the tumour – the more advanced the cancer, the higher the expression [22, 23]. Therefore, the pattern of 4-HNE histological appearance is dependent on the histological origin of cancer and is not universal [24].
Cellular lipids comprise a large family of saturated fatty acids, PUFAs, monounsaturated fatty acids, phospholipids, and many more [25]. High-risk HPV strains related to CC generate energy through the process of aerobic glycolysis, also known as the Warburg effect, as opposed to oxidative phosphorylation, which is the preferred method for ATP synthesis by the cell [25]. Human papilloma virus 16 oncoproteins E6 and E7 favour the Warburg effect, also increasing lipid and protein synthesis [25].
Another interesting metabolic switch in HPV-related cancers is the production of energy through β-oxidation. Prusinkiewicz et al. showed that HPV-related cancers induce β-oxidation of fatty acids to generate energy in the form of ATP [26]. β-oxidation, on the other hand, generates ROS in the form of hydrogen peroxide, which can damage the mitochondria if it is not eliminated [27]. When damaged, the electron transport chains in the mitochondria do not function properly, generating more ROS such as hydroxyl and superoxide radicals. Reactive oxygen species, as mentioned above, bind to PUFAs, causing lipid peroxidation and thus generating highly reactive second messengers such as 4-HNE. By altering its metabolism, CC sustains the production of ATP and other macromolecules needed for cell growth, division, and survival, which might explain the increased expression of second messengers, such as 4-HNE in the more advanced stages of the disease. However, to better evaluate the role of 4-HNE in CC progression, a larger sample of patients is needed for further investigation.
The higher expression of 4-HNE in metastatic lymph nodes can be explained by the epithelial-mesenchymal transitions (EMT) induced by HPV16 oncoproteins E6 and E7 [25]. Saturated fatty acid replacement with PUFAs has been proven to play a critical role in both EMT and metastasis [25]. Saturated fatty acids are prevalent in locally grown tumours, but PUFAs are more prevalent at the metastatic stage, promoting tumour invasion and spread [25]. E6 and E7 increase the levels of vimentin and E-cadherin in the cell, which in turn promotes EMT and metastasis [25].
Conclusions
4-hydroxynonenal produced through lipid peroxidation is not only a cytotoxic compound but also an important asset in cancer signalling pathways. The biological activity of this second messenger depends on the histological type of the cancer, the levels of antioxidants in the body, the exogenous and endogenous production of fatty acids, and so on. Many studies have shown the importance of 4-HNE as a biomarker for oxidative stress and its role in cancer initiation and development. To the best of our knowledge, this is the first study testing the expression of 4-HNE in CC using immunohistochemistry. The three score systems showed similar results with no significant differences. The expression of 4-HNE was higher in FIGO stage 3 samples compared to early stages of the disease. There was also a statistically significant difference in patients with metastatic lymph nodes compared to those without. The limitation of our study is the relatively small sample of patients taking part – only 69. Further studies are required to evaluate the role of 4-HNE in CC progression.