Purpose
Mediastinum is a common site that harbors specific malignant tumors. Most patients with mediastinal metastases lose the chance of surgery, with chemotherapy and radiotherapy being the classic treatments. Brachytherapy is a radiotherapy technique, in which radioactive sources are precisely placed into or next to tumors, aiming at safe delivery of high doses of radiation to eliminate and shrink tumors without affecting surrounding tissues, since radiation doses rapidly decline with increasing distance from the source [1, 2]. In contrast, intensity-modulated radiation therapy (IMRT) is an external beam form of radiotherapy that uses three-dimensional radiotherapy technology, which aims to improve local control rate of tumor development and reduce damage to adjacent normal tissues [3]. In the literature, both approaches have been compared mainly for prostate cancer treatments and to date, brachytherapy has been reported to be superior to IMRT [4-7]. The efficacy and safety of computed tomography (CT)-guided 125I implantation for the treatment of mediastinal metastases and primary mediastinal malignant tumors has also been proven [8-10]. However, the anatomical structures in the mediastinum are complex, and lesions are often adjacent to the superior vena cava, aorta, and other great blood vessels. Furthermore, there are many structures, such as the esophagus, sternum, and thoracic vertebra in the puncture path, which potentially surround target lesions. In addition to these, the percutaneous puncture needs to pass through much of the lung tissue because the distance from mediastinal lesions to the chest wall is quite long. Therefore, a great risk of puncture exists, and it is also difficult to meet the requirements of pro-plan. In some cases with no other structures between lesions and the sternum, the use of a trans-sternal approach is safe for CT-guided biopsy of the anterior or middle mediastinal nodes [11, 12]. However, in contrast to CT-guided percutaneous needle approaches for intra-thoracic lesions, there are few reports about CT-guided trans-sternal 125I seed implantations. Encouraged by these reports, we performed CT-guided trans-sternal 125I seeds implantation in a cohort of patients with anterior and middle mediastinal metastasis. The aim of the study was to retrospectively evaluate the feasibility and clinical value of this alternative treatment technique.
Material and methods
Ethics
This study was approved by the ethics committee of The First Affiliated Hospital of Zhengzhou University. All included patients provided informed written consent before taking part in the study.
Patients
From September, 2017 to December, 2019, twenty patients (12 males and 8 females; mean age, 55.50 ±14.57 years, range, 16-85 years) with anterior or middle mediastinal metastasis underwent CT-guided trans-sternal 125I seed implantation (Figure 1). Inclusion criteria were all patients with malignant tumor diagnosed by histology, with metastases confirmed by further histology or radiographic options (enhanced CT or MRI, PET-CT); well-controlled primary tumor; patients who failed or refused to undergo chemotherapy, external radiotherapy, or surgery; lesions of < 7 cm in dimension; expected survival time of > 3 months; Karnofsky performance scale (KPS) scores of ≥ 70. Characteristics of the patients are listed in Table 1.
Fig. 1
Patients with anterior or middle mediastinal metastasis underwent CT-guided via trans-sternal 125I seed implantation
Table 1
Basic characteristics and dose parameters in pre-operative and post-operative plans of 20 patients, and their clinical responses after 125I implantation
Instruments and equipment
CT (CT590, GE, USA), 125I seed (HTA, Beijing, China; Seeds Biological Pharmacy, Tianjin, China; half-life of 59.6 days; average energy of 27.4-35.5 kV; length: 4.5 mm; diameter: 0.8 mm; half layer; 0.025 mm of lead), Hakko interventional steel puncture needle (18 G, 15 cm), and a brachytherapy treatment planning system (BTPS, Beijing Astro Technology, Beijing, China) were used in the study.
Procedures for 125I implantation
All procedures were performed by the same interventional radiologist ZG Zhou, who has 20 years of experience in CT-guided percutaneous interventions. Contrast material-enhanced CT was performed in each patient, up to 7 days before surgery.
Pre-operative treatment plan was developed within 3 days of surgery using BTPS by two radiologists (T. Liu and Y.L. Chen). Gross tumor volume (GTV), planned target volume, and surrounding vital structures were measured based on the contrast material-enhanced CT images. BTPS simulated the needle path and calculated the number of seed. The prescribed dose per volume was 110-140 Gy.
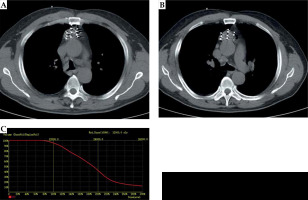
BTPS generated a dose-volume histogram (DVH) and isodose curves of different percentages. Great care was taken to ensure that all needle paths avoided vital structures, including large blood vessels (Figure 2). On the day of surgery, a patient was placed on the CT gantry, and routine CT imaging (parameters: voltage, 120 kV, 200 mA per section; section thickness, 5 mm; rotation time, 0.75 s) was performed to locate the lesion site. After local infiltration, anesthesia with 5-20 ml of 1% lidocaine (Hunan Kelun, Yueyang, China) was performed, and 18-gauge needles were used to directly puncture the sternum under CT guidance, based on pre-operative plan (Figure 3). The needles were twirled gradually and positioned against the lesions’ deepest margins. Intra-operative real-time plan was performed based on positions of the needles and 125I seed distribution. After completion of surgery, a routine chest CT scan was taken immediately to confirm the presence or absence of complications, such as pneumothorax and hemorrhage. Dose evaluation was performed based on the recorded images (Figure 4).
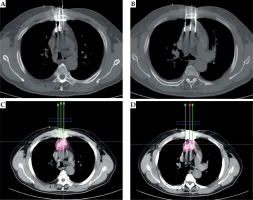
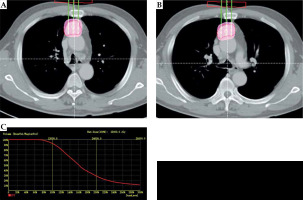
Fig. 2
Case 1: A 61-year-old male with lung squamous cell carcinoma. Pre-operative plan (A, B). BTPS simulated needle path, and seed number and distribution were calculated; the DVH (C). Prescribed dose was 120 Gy, D90 was 136.88 Gy, and V100 was 95%
Follow-up and evaluation criteria
Any complications were noted based on the CT images taken immediately after surgery. Dose parameters of the pre-operative and post-operative plans, including D90, V90, V100, V150, V200, mean conformity index (CI), external index (EI), and homogeneity index (HI) were carefully recorded. D90 was defined as the dose received by 90% of target volume, and V100 was defined as the percentage of target volume receiving 100% of the prescribed dose. A D90 of at least 90% of the prescribed dose and a V100 that corresponded to at least 90% of the target volume is the recommended standard of care. V150 and V200 corresponded to the target volumes receiving 150% and 200% of the prescribed dose [13]. When the actual dose delivered and identified met the pre-operative planed standard of care, it was considered to be a satisfactory outcome. Contrast material-enhanced CT images were obtained at 2- to 3-month intervals within 1 year of surgery, and then at 3-month to 6-month intervals. The sum of the longest dimensions was recorded as the size of target lesion. Local therapeutic efficacy was evaluated according to the size of tumor. Complete response (CR) was defined as disappearance of the target lesion, or only accumulative 125I seed shadows were detectable by image analysis (Figure 5). Partial response (PR) was evaluated as more than a 30% decrease in the dimension of target lesion, taking baseline as a reference (Figure 6). Stable disease (SD) was defined as neither PR nor progressive disease (PD), and PD as more than a 20% increase in the dimension of target lesion, taking as reference the smallest dimension from the time the treatment was initiated.
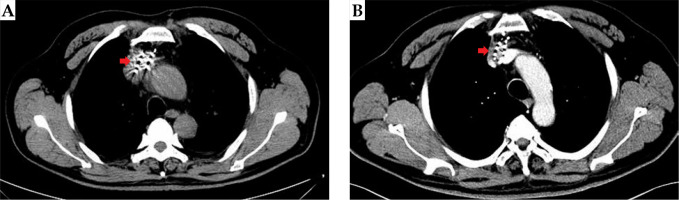
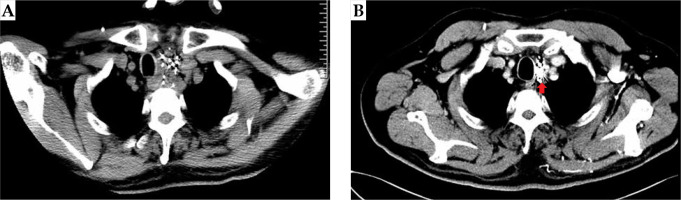
Fig. 5
Case 2: A 53-year-old patient with mediastinal metastatic lymph node recurrence from esophageal squamous cell carci- noma. 125I seeds distribution immediately after the surgery (A); Disappearance of the target lesion and only accumulative 125I seed shadows were detectable by image analysis (arrow) (B); The local therapeutic efficacy was complete response
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 (IBW, NY, USA). The measured data were presented as mean ± standard deviation. Wilcoxon signed-rank test was used to analyze the data, and differences were considered statistically significant if p-values were < 0.05.
Results
Table 1 shows the differences in dose parameters between post-operative and pre-operative plans. A total of 22 lesions were treated with 125I implantation in twenty patients. All the procedures were successfully completed. The mean number of implanted 125I seeds was 31.05 ±18.94 (range, 6-84 seeds). The mean D90, V90, V100, V150, V200, CI, EI, and HI were 134.30 ±14.53 Gy, 96.1 ±1.55%, 92.69 ±1.93%, 66.86 ±7.53%, 42.95 ±9.11%, 0.65 ±0.06%, 40.79 ±13.72%, and 27.90 ±7.53%, respectively. Statistically significant differences were found for D90, V90, V100, and V200, with p = 0.015, 0.013, 0.009, and 0.014, respectively (Table 1). The 125I seed distribution of two patients did not meet the requirements. D90 values of those patients were 109.69 Gy and 109.21 Gy, which were lower than the prescribed dose. The V100 values of the 2 patients were 89.8% and 89.7%, respectively, which were < 90%. The satisfaction rate of 125I seeds distribution was 90%.
From the time of 125I implantation, the mean follow-up duration was 12 ±4.75 months (range, 4-24 months). The local control rates at 2 months, 6 months, and 1 year after surgery were 65.0%, 64.7%, and 53.8%, respectively. Table 1 demonstrates local efficacy at 2 months after 125I seeds implantation. Three patients locally relapsed, and 4 patients died, but no radiation-related complications occurred throughout the follow-up period. Clinical responses of patients to 125I implantation are summarized in Table 1.
Regarding toxicity, one patient had a small amount of pneumothorax, and another one had hemoptysis after surgery. No fatal complications occurred within 24 hours after intervention, and no serious complications were observed during follow-up. There was no radiation pneumonia, radiation esophagitis, or radiation skin reaction during the follow-up period.
Discussion
In recent years, CT-guided 125I seeds implantation has been used to treat various malignant solid tumors, including primary metastatic lung cancer, pancreatic, primary or metastatic liver cancer, prostate cancer, and soft-tissue sarcoma as well as salvage therapy for cervical lymph node recurrence after external beam radiation of esophageal squamous cell carcinoma. This approach offers many advantages, such as a high local control rate and good therapeutic effect with minimal injury to normal tissue, with precise anatomical location and shorter hospital stay compared to routine radiotherapy [14-23]. But there are limits for a successful treatment of mediastinal masses because of the complex anatomical structure involved: 1. The approach of masses in the anterior or middle mediastinum can be challenging because they are often adjacent to the great blood vessels; 2. The puncture needle path is blocked by the sternum; 3. Transpulmonary is the traditional approach, but pneumothorax and hemorrhage are common complications of CT-guided 125I seeds implantation; 4. It can be difficult to meet the required dose parameters of pre-operative plan. Thus, the application of 125I seeds for the treatment of patients with masses in the mediastinum is limited. In order to decrease the incidence of complications and increase the satisfaction rate for 125I seed distribution, Lin et al. used non-transpulmonary puncture interstitial implantation of 125I seeds to treat recurrent mediastinal lymph node metastasis via parasternal, paraspinal, suprasternal, and trans-sternal approaches [24]. Only one patient experienced a small amount of pleural cavity bleeding. For some patients with metastases in the middle or upper posterior, trans-superior vena cava and trans-tracheal approaches were also reported to be safe by Liu and Lei [25-27]. It is well-known that via the sternum is considered to be a safe approach for CT-guided percutaneous needle biopsy of mediastinal nodules [2, 11]. In our study, we used the sternum approach for CT-guided 125I seeds implantation to treat twenty patients with masses in the anterior or middle mediastinum. Only 1 patient experienced hemoptysis and 1 experienced pneumothorax, both of them cured by a conservative treatment. No serious complications were noted. A CT-guided via the sternum approach for 125I seeds implantation for the treatment of lesions in the anterior or middle mediastinum has many advantages: 1. The distance of the puncture path is short; 2. There is a noticeable decrease in the incidence of lung injury; 3. Most of the needles can be arranged parallelly to each other.
The efficacy and safety of 125I implantation depends on the precision of puncture and dose distribution in the target area [28, 29]. Therefore, reasonable quality control of dosimetry is an important step during 125I seeds implantation [30]. A pre-operative plan and dosimetry verification should be performed in TPS before and immediately after the implantation. In recent years, intra-operative real-time planning was successfully implemented in many centers, and has been used to treat prostatic cancer, lung cancer, retro-peritoneal metastatic cancer, and gliomas [31-34]. It can improve the accuracy of 125I seed placement and consistency of dose distribution between post-operative and pre-operative stages. Hence, in the present study, we used intra-operative real-time planning. Immediately after surgery, the post-operative plan was performed for dose evaluation. Prescribed doses of 110-140 Gy were chosen according to previously published dose ranges [13]. Two patients in our study did not receive the required dose. D90 of the 2 patients was 109.69 Gy and 109.21 Gy, while a pre-plan was to receive 110 Gy for both of them. V100 of the 2 patients was 89.8% and 89.7%, respectively, which was somewhat less than the threshold of 90% for standard of care. Dose parameters post-operatively met the requirements of the pre-plan. Despite this finding, there were statistically significant differences in D90, V90, V100, and V200, with p-values = 0.015, 0.013, 0.009, and 0.014, respectively, between the post-operative and pre-operative plans. Some important factors to consider when formulating an intra-operative plan: 1. The positions of needles in the plan should be the same as in the real world; 2. 125I seeds should be implanted according to intra-operative real-time plan; 3. If the dose did not meet pre-operative plan, 125I seeds should be replanted immediately.
Several studies have confirmed that CT-guided 125I seed implantation for mediastinal tumors had a superior local control rate [35-37]. Gao et al. reported that CT-guided 125I implantation was an effective and safe treatment for mediastinal metastatic lymph node recurrence in esophageal carcinoma. Local control rates of 3, 6, 10, and 15 months after surgery were 75.0%, 50.0%, 42.9%, and 33.3%, respectively [36], which are comparable to our findings. In another study, Xu et al. reported endobronchial ultrasound-guided iodine-125 radioactive seed implantation for a treatment of mediastinal tumors. They found that overall response rates (complete remission + partial remission) post-operatively at 2, 4 and 6 months were 65% (13/20), 80 (16/20), and 85% (17/20), respectively [38]. Another study reported a local control rate of 40.6%, 1 year after (CT) guided 125I seed implantation for recurrent head and neck cancer after external beam radiation therapy [39], which is also close to the value of 53.8% found in the present study.
This study had a number of limitations. It included only twenty patients, who were followed-up for less than 2 years. Future studies should involve a larger cohort of patients and longer follow-up time. CT-guided 125I seed implantation is a local treatment modality, which should be used in conjunction with effective systemic treatment.