Urticaria is characterized by recurrent itchy wheals and/or angioedema and is usually classified as chronic urticaria (CU), if clinical symptoms persist for more than 6 weeks. In spontaneous CU, by definition, no specific triggers are associated with the appearance of signs and symptoms.
One of the main underlying mechanisms responsible for urticaria is the activation of skin mast cells, which release histamine and other pro-inflammatory mediators involved in wheal formation and itch [1]. Aside from mast cells, other histamine-bearing cells exist, such as basophils, endothelial cells, gut cells, brain cells, smooth muscle cells and platelets. In particular, platelets contain a broad range of biological mediators: preformed mediators, such as histamine and serotonin, and newly formed mediators, such as platelet activating factor (PAF), leukotrienes and prostaglandins [2].
Anti-histamines are widely used to treat urticaria because of their ability to block histamine receptors, resulting in amelioration of histamine-related symptoms. In fact, some anti-histamines can also interfere with platelet-associated mediators. As an example, rupatadine induces the blockage of both histamine receptors and PAF receptors [3]. Likewise, cyproheptadine has also an anti-serotonin effect [4].
Essential thrombocythemia (ET) is a rare chronic blood condition, characterized by overproduction of thrombocytes by the megakaryocytes, in the bone marrow. Clinical symptoms of ET are weakness, chest pain, fainting, temporary vision changes, headache, numbness and tingling of hands and feet. According to the 2016 WHO guidelines, ET diagnosis relies on the following criteria: i) platelets count ≥ 450 × 109/l; ii) bone marrow biopsy showing proliferation mainly of the megakaryocyte lineage, without any significant increase in granulopoiesis or erythropoiesis and minor increase in reticulin fibres; iii) exclusion of BCR-ABL mutation, chronic myelogenous leukaemia, polycythemia vera, primary myelofibrosis, myelodysplastic syndrome and other myeloid neoplasms; iv) presence of a gene mutation for either Janus Kinase 2 (JAK2) or calreticulin or thrombopoietin receptor; and v) absence of evidence for reactive thrombocytosis [5].
In particular, the mutation V617F in the Jak2 gene (JAK2V617F) was found in 23–57% of patients suffering from ET. This mutation encodes for a constitutively phosphorylated tyrosine kinase, which results in permanent cell activation and abnormal proliferation of megakaryocytes and thrombocytes [6]. The gold standard treatment for ET is hydroxyurea (HU) that inhibits the G1/S phase of the cell cycle and reduces megakaryocyte proliferation [7]. Thus, a drop in platelet counts is induced.
Here we report the case of an adult patient affected by CU that eventually revealed an underlying ET. A 62-year-old woman, without previous clinical history of allergy, was referred to our Clinic in September 2019 for CU, which had started in May 2019. She had undergone a treatment with desloratadine 5 mg (1 tablet daily), which nonetheless had no appreciable effect (Figure 1 A). At first, in order to achieve control of clinical symptoms we doubled the desloratadine dosage (5 mg/twice a day) achieving a fair control of CU and the related symptoms. However, in January 2020, the CU relapsed and became unresponsive to desloratadine therapy.
Figure 1
A – Urticaria activity score 7 (UAS-7) in the ET patient. B – Platelet counts: ET patient (circles); 121 severe CU controls (crosses)
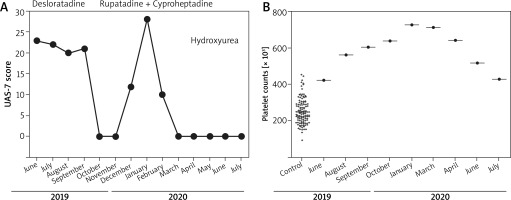
Meanwhile we performed a thorough allergological workup for CU. Thus, we assessed autoimmunity by evaluating antinuclear and anti-thyroid gland antibodies, which proved negative. Interestingly, a steady increase in platelet counts was detected through haemocytometric tests performed in June, August, September and October 2019 (424 × 103 cells/μl, 562 × 103 cell/μl, 605 × 103 cell/μl, 639 × 103 cell/μl, respectively) (Figure 1 B).
Considering the CU relapse and the increase in platelet counts, we hypothesized that the thrombocytosis and the CU activity might be related to each other. Therefore, we switched to a treatment approach, aiming at interfering with platelet-derived mediators: rupatadine 10 mg, daily, and cyproheptadine 5 mg, daily. By this treatment we achieved a complete symptom resolution (Figure 1 A). Of note, we also analysed the blood counts of 121 patients suffering from severe CU and found out that their platelet counts were not altered (mean: 253.5 ±68.2 × 103 cells/μl), suggesting that CU, by itself, does not induce reactive thrombocytosis. After a haematological consult, 7.83% of JAK2 V617F mutation was found in peripheral blood (Ipsogen JAK2 MutaQuant Kit CE, Qiagen, Germany). Moreover, the bone marrow biopsy revealed a histological picture compatible with ET (increase in megakaryocyte number and size, with an altered morphology). Thus, in May 2020, the patient started the treatment with HU. Since the beginning of this treatment, blood tests showed decreased platelet counts (Figure 1 B), as expected. Eventually, the anti-histamine treatment could be discontinued with no CU relapse.
In this report, for the first time we describe a patient suffering from CU possibly related to an underlying ET. In fact, platelets have been reported to be involved in various inflammatory conditions as well as in experimental models of anaphylaxis [8]. However, their role in CU has been poorly investigated.
The case presented shows that CU can also be related to platelet homeostasis dysregulation, as suggested by the increase in platelet counts mirroring the 7-day urticaria activity score (UAS 7) curve (Figures 1 A, B). Moreover, the therapeutic strategy adopted, including HU, a drug directly affecting megakaryocyte proliferation, along with an anti-PAF agent (rupatadine) combined with an anti-serotonin agent (cyproheptadine), led to complete resolution of CU symptoms. On the other hand, it is unlike that the increase in platelet counts observed in our patient was due to reactive thrombocytosis, secondary to CU, since in 121 patients suffering from severe CU, the platelet counts were in the normal range (Figure 1 B).
In conclusion, we propose that: i) platelet count is a parameter to be routinely considered in the diagnostic workup of CU; ii) an elevated platelet count should be taken into account in choosing the right anti-histamine in CU treatment; iii) CU might represent an early presentation of ET; and iv) treatment with HU induces a good clinical control of ET-related CU.








