Introduction
Acute biliary pancreatitis (ABP) is an acute abdomen condition caused by biliary tract diseases that trigger outflow obstruction and hypertension of the pancreatic duct and subsequent pancreatic autodigestion to result in pancreatic edema, hemorrhage, and necroinflammatory injury [1]. It is characterized by acute epigastric pain and elevated serum amylase or lipase, often with clinical symptoms such as abdominal pain and distension, nausea and vomiting, fever, and gastrointestinal bleeding. Most symptoms of ABP patients are mild and self-limiting; however, about 20% to 25% may experience local or systemic complications and develop severe biliary pancreatitis or even necrotizing pancreatitis and multiple organ failure, with a mortality rate of about 2% to 10% [2, 3]. The pathogenesis of ABP is complex and diverse, with gallstone-caused obstruction and biliary tract infection being the main causes of ABP attacks, and conservative treatments or surgical treatments are advocated [4, 5]. Conservative treatments such as fluid resuscitation and gastrointestinal decompression are usually used for organ support and control of inflammation.
With the continuous development and update of endoscopic technologies, endoscopic retrograde cholangiopancreatography (ERCP) has become the most vital diagnosis and treatment in pancreatic and biliary diseases and is widely used in clinical practice. With the combination of abdominal ultrasound with endoscopic ultrasonography, ERCP can diagnose ABP by finding gallstones in duodenal fluid or bile. Additionally, with the combination of endoscopic sphincterotomy (EST), this technique can also be used to incise the sphincter of Oddi to relieve pancreatic hypertension, contributing to better treatment efficacy and outcomes [6–9]. However, it is essential to standardize the indications for ERCP, and some studies suggest that using ERCP hurriedly could lead to poor clinical efficacy and thus delay the condition in case of no diagnosis of gallstones in ABP patients [10]. Others have proposed that the application of ERCP does not significantly improve the therapeutic benefit of ABP patients based on the analysis of mortality and medical costs, and believe that possibly some ABP cases are overtreated by ERCP. In a meta-analysis performed in 2008, the risk of overall complications and mortality was not significantly reduced after early ERCP intervention in patients with acute pancreatitis without cholangitis [11]. Increasing numbers of relevant studies have been published, but their results are conflicting [6, 12].
Aim
Given the controversial conclusion on the application of ERCP, this study aimed to systematically investigate the clinical efficacy of ERCP or ERCP combined with EST in the treatment of ABP. Specifically, through a meta-analysis of relevant randomized controlled trials (RCTs), changes in serum biochemical parameters and inflammatory factor levels after treatment were compared between the control group (conservative treatment) and the observation group (ERCP or ERCP + EST on the basis of the control group), to provide data support and guide clinical application of ERCP or ERCP combined with EST in the treatment of ABP.
Material and methods
Literature search strategy
A literature search was performed in PubMed, Web of Science, China Knowledge Infrastructure, and Wanfang databases for related articles published from January 2010 to January 2022. The keywords were “endoscopic retrograde cholangiopancreatography”, “acute biliary pancreatitis”, and “treatment”. This meta-analysis was performed following the PRISMA guidelines.
Inclusion criteria
(1) Study type: RCTs comparing the clinical efficacy of conservative treatment versus ERCP treatment in patients with ABP; (2) Study subjects: Patients diagnosed with ABP based on their clinical manifestations, laboratory results such as serum amylase and lipase, imaging examinations such as abdominal ultrasound and computed tomography; (3) Intervention measures: The control group received conservative treatment, while the observation group received ERCP or ERCP combined with EST on the basis of conservative treatment; (4) Outcome measures: Response rate after treatment, incidence of complications, abdominal pain relief time, intestinal exhaust recovery time, length of hospital stay, serum amylase recovery time, total bilirubin (Tbil), white blood cell count (WBC), serum C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α).
Exclusion criteria
(1) Studies with a design type of non-RCT, no clear diagnostic criteria, and no intervention of ERCP or ERCP combined with EST; (2) Studies with vague and missing data which could not be converted and combined, and the key data could not be obtained after communication with the authors of the literature; (3) reviews, case reports, conference papers and other non-article materials.
Literature screening and data extraction
Duplicate literature was eliminated with the EndNote software and manual checking, and two researchers independently screened the remaining literature according to the inclusion criteria and exclusion criteria. During literature screening, a third investigator participated in the discussion in cases of disagreement between the two researchers to reach a final consensus. The following data were extracted: title, first author, publication time, study design, sample size and basic characteristics of study subjects, intervention methods, outcome measures, and other detailed information. The risk of bias was assessed using Review Manager 5.30 according to the Cochrane Handbook for Systematic Reviews of Interventions.
Statistical analysis
The Stata 16.0 statistical software was used to analyze the relevant data of the included articles, with odds ratio (OR) used to express binary variables and standardized mean difference (SMD) with 95% confidence intervals (CI) to express continuous variables. The statistical heterogeneity among studies was evaluated using the χ2 test and I2 statistic. P > 0.05 and I2 < 50% indicated no significant difference in heterogeneity, so the fixed-effects model was selected. Otherwise, the random-effects model was selected for meta-analysis. The reliability of the analysis results was verified by sensitivity analysis. If the number of included studies was more than 10, a funnel plot was made using the Begg method for publication bias analysis. P < 0.05 indicated statistically significant results.
Results
Literature search results
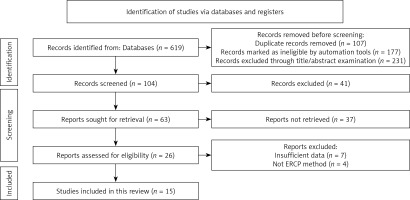
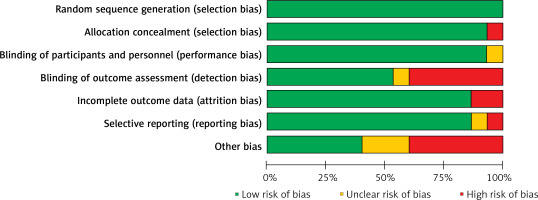
In total, 619 studies were preliminarily retrieved, of which 284 were excluded due to being duplicates or ineligible. Then, after screening for titles and abstracts, 231 studies were excluded, and an additional 89 articles were excluded after reading the original text. Finally, 15 RCTs were included in the study [6, 13–26], consisting of 1639 ABP patients (816 in the control group and 823 in the observation group). The detailed screening process and results are shown in Figure 1. The basic characteristics of the included RCTs are displayed in Table I. The risk of bias evaluation is shown in Figures 2 and 3.
Table I
Basic characteristics of included literature
| First author and publication year | Group | n | Age [years] | Gender(male/female) | Intervention | Study design | Outcome measures |
|---|---|---|---|---|---|---|---|
| Zhou Dan 2019 [14] | Ob group | 73 | 56.5 ±11.0 | 40/33 | CT + ERCP | RCT | ①③④⑤⑦⑧⑨⑩ |
| Co group | 73 | 57.2 ±11.4 | 38/35 | CT | RCT | ||
| Zhai Qizhi 2011 [25] | Ob group | 22 | 45.7 ±8.4 | 10/12 | ERCP | RCT | ②③④⑤⑥⑧ |
| Co group | 20 | 43.7 ±7.5 | 10/10 | CT | RCT | ||
| Lu Qiuliang 2014 [23] | Ob group | 40 | 45.6 ±4.7 | 31/9 | CT + ERCP + EST | RCT | ②③④⑤⑥⑧⑨⑩ |
| Co group | 40 | 46.9 ±5.2 | 32/8 | CT | RCT | ||
| Zhang Chao 2020 [18] | Ob group | 50 | 34–69 | 32/18 | ERCP | RCT | ③④⑥⑦⑧⑨⑩ |
| Co group | 50 | 35–70 | 31/19 | CT | RCT | ||
| Zhong Canxin 2020 [15] | Ob group | 55 | 53.39 ±6.27 | 32/23 | CT + ERCP | RCT | ②⑨ |
| Co group | 55 | 54.26 ±6.33 | 30/25 | CT | RCT | ||
| Zhao Guang 2017 [16] | Ob group | 34 | 45.8 ±4.3 | 21/13 | ERCP + EST | RCT | ①②③⑤⑥⑨⑩ |
| Co group | 34 | 45.9 ±3.9 | 20/14 | CT | RCT | ||
| Ma Xuefei 2021 [26] | Ob group | 30 | 57.43 ±3.34 | 15/15 | ERCP | RCT | ①③④⑤⑥⑦⑧ |
| Co group | 30 | 56.52 ±3.51 | 15/15 | CT | RCT | ||
| Niu Jingwei 2019 [22] | Ob group | 100 | 68.87 ±4.03 | 62/38 | ERCP | RCT | ①③④⑤⑥⑨⑩ |
| Co group | 100 | 69.26 ±4.21 | 64/36 | CT | RCT | ||
| Lai Jiawen 2019 [24] | Ob group | 34 | 42.3 ±15.7 | 12/22 | ERCP | RCT | ②③④⑤⑥⑧ |
| Co group | 40 | 40.0 ±13.2 | 16/24 | CT | RCT | ||
| Nong Wei 2021 [21] | Ob group | 50 | 46.54 ±5.23 | 36/14 | ERCP | RCT | ①③⑤⑥⑦⑧⑨⑩ |
| Co group | 50 | 45.39 ±4.78 | 39/11 | CT | RCT | ||
| Zhang Guoping 2018 [17] | Ob group | 48 | 38–74 | 21/27 | ERCP | RCT | ①②③⑤⑥⑦⑧ |
| Co group | 37 | 32–69 | 16/21 | CT | RCT | ||
| Tang Chenglu 2018 [20] | Ob group | 70 | 55.4 ±7.9 | 41/29 | ERCP | RCT | ②③④⑤⑦⑨⑩ |
| Co group | 69 | 56.2 ±8.3 | 39/28 | CT | RCT | ||
| Xu Qinlong 2021 [19] | Ob group | 50 | 49.96 ±3.15 | 26/24 | ERCP + EST | RCT | ③④⑥⑨⑩ |
| Co group | 50 | 49.87 ±3.62 | 27/23 | CT | RCT | ||
| Nicolien J Schepers 2020 [6] | Ob group | 117 | 69 ±13 | 66/51 | ERCP + EST | RCT | ①② |
| Co group | 113 | 71 ±12 | 60/53 | CT | RCT | ||
| Zhou Wence 2011 [13] | Ob group | 50 | 52.7 ±15.4 | 22/28 | ERCP + EST | RCT | ①②⑤⑦⑧⑩ |
| Co group | 55 | 58.3 ±13.3 | 26/29 | CT | RCT |
[i] Ob group – observation group, Co group – control group, CT – conservative treatment, ERCP – endoscopic retrograde cholangiopancreatography, EST – endoscopic sphincterotomy, RCT – randomized controlled trial, ① response rate, ② incidence of complications, ③ abdominal pain relief time, ④ intestinal exhaust recovery time, ⑤ length of hospital stay, ⑥ serum amylase recovery time, ⑦ total bilirubin, ⑧ white blood cell count, ⑨ serum C-reactive protein, ⑩ tumor necrosis factor-α.
Clinical efficacy of ERCP in the treatment of ABP
Meta-analysis results of response rate and incidence of complications
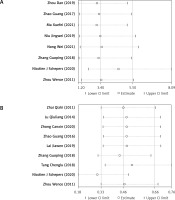
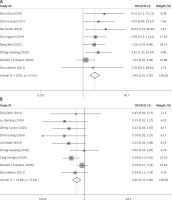
Among the RCTs included in this meta-analysis, 8 reported on response rate after ERCP, and no significant heterogeneity was detected among the studies (I2 = 0.0%, p = 0.535). Using the fixed-effects model, the analysis results showed that the response rate in the observation group was higher than that of conservative treatment alone (OR = 3.399, 95% CI: 2.100, 5.499, p < 0.001; Figure 4 A), and the difference was statistically significant. In addition, 9 studies reported on the incidence rate of postoperative complications, with no significant heterogeneity detected among the studies (I2 = 22.8%, p = 0.241). The analysis using the fixed-effects model showed that patients presented with a lower incidence of complications after ERCP (or ERCP + EST) compared with those receiving conservative treatment (OR = 0.463, 95% CI: 0.326, 0.658, p < 0.001; Figure 4 B), and the difference was statistically significant.
Figure 4
Forest plots comparing response rate (A) and incidence of complications (B) between the two groups
Further, the source of heterogeneity was found by sensitivity analysis to ensure the reliability of the results. The sensitivity analysis results of the response rate and incidence of complications of the included literature are shown in Figures 5 A and B, respectively. The fixed-effects model was used for calculating the two outcome measures, and the obtained p-value, I2 value and OR were consistent with the original meta-analysis results, indicating good stability of the analysis results.
Meta-analysis results of prognostic indicators after treatment
Twelve studies involved abdominal pain relief time after treatment, 9 reported intestinal exhaust recovery time, 10 mentioned the time for serum amylase to return to normal, and 11 reported the length of hospital stay. Significant heterogeneity was observed among the studies (abdominal pain relief time, I2 = 90.1%, p = 0.001; intestinal exhaust recovery time, I2 = 90.9%, p = 0.001; serum amylase recovery time, I2 = 95.4%, p = 0.001; length of hospital stay, I2 = 88.1%, p = 0.001), so the random-effects model was used for combined analysis.
The results showed that after treatment, the observation group had significantly lower abdominal pain relief time (SMD = –2.288, 95% CI: –2.756, –1.821, p < 0.001; Figure 6 A), intestinal exhaust recovery time (SMD = –1.837, 95% CI: –2.347, –1.328, p < 0.001; Figure 6 B), serum amylase recovery time (SMD = –2.174, 95% CI: –2.969, –1.379, p < 0.001; Figure 6 C), and hospital stay (SMD = –1.765, 95% CI: –2.180, –1.349, p < 0.001, Figure 6 D), and the differences between the two groups were statistically significant.
Figure 6
Forest plots comparing the prognostic indicators after treatment between the two groups: A – abdominal pain relief time, B – intestinal exhaust recovery time, C – serum amylase recovery time, D – length of hospital stay

For sensitivity analysis, the data were logarithmically transformed using the random-effects model, and individual studies were excluded. Sensitivity analysis of abdominal pain relief time, recovery time of intestinal exhaust, recovery time of serum amylase, and length of hospital stay are shown in Figures 7 A–D. The obtained p-value, I2 value, and SMD were consistent with the original meta-analysis results, and no marked difference was identified between the two results, indicating that the meta-analysis results had good stability.
Figure 7
Sensitivity analysis of prognostic indicators after treatment in the two groups (A) abdominal pain relief time; (B) intestinal exhaust recovery time; (C) serum amylase recovery time, (D) length of hospital stay

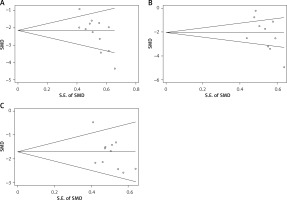
Subsequently, publication bias analysis was performed for the abdominal pain relief time, serum amylase recovery time and hospital stay time, and the results are shown in Figures 8 A–C, respectively. In these funnel plots, the scatter points on both sides were distributed at the bottom and in an asymmetric manner, indicating that the included studies might have some publication bias, which was possibly related to the small sample size or low quality of some included studies.
Meta-analysis of serum biochemical indicators and inflammatory factors after treatment
Among the included studies, 7 compared Tbil levels in ABP patients after treatment and 9 compared WBC, CRP and TNF-α levels. Significant heterogeneity was found among the studies (Tbil, I2 = 97.6%, p = 0.001; WBC, I2 = 87.0%, p = 0.001; CRP, I2 = 96.8%, p = 0.001; TNF-α, I2 = 94.7, p = 0.001), so random-effects model analysis was used. The results showed that compared with the control group, the observation group had better performance in the levels of Tbil (SMD = –2.471, 95% CI: –3.633, –1.310, p < 0.001; Figure 9 A), WBC (SMD = –1.304, 95% CI: –1.742, –0.866, p < 0.001; Figure 9 B), CRP (SMD = –2.963, 95% CI: –3.915, –2.011, p < 0.001; Figure 9 C), and TNF-α (SMD = –4.822, 95% CI: –5.806, –3.839), p < 0.001; Figure 9 D).
Figure 9
Forest plots comparing serum biochemical indicators and inflammatory factors after treatment between the two groups: A – total bilirubin, B – white blood cell count, C – serum C-reactive protein, D – tumor necrosis factor-α

With logarithmic transformation using the random-effects model, sensitivity analysis was performed for Tbil, WBC, CRP and TNF-α levels (Figures 10 A–D) by eliminating individual studies one by one. The obtained p-value, I2 value and SMD were consistent with the original meta-analysis results and showed no difference between the two results, indicating that the meta-analysis results had good stability.
Discussion
In the course of ABP, persistent hypertension of the biliopancreatic ampulla is the main cause of severe ABP [27, 28]. Therefore, early relief of biliopancreatic duct obstruction is essential to treating ABP. Such relief can prevent bile reflux into the pancreatic duct, resulting in pancreatic secretion of trypsin, activation of related inflammatory factors, multiple organ function injury or even failure.
Although ERCP was first reported by William et al. in 1968, and Classen and Demling in Germany and Kawaia et al. in Japan performed the first EST in 1974, it was not widely accepted until the advent of a new generation of duodenoscopes [29–31]. ERCP is generally performed as follows: first, the endoscope is passed through the upper gastrointestinal tract into the duodenum; second, the catheter is retrogradely inserted through the opening of the duodenal papilla to gain access to the common bile duct and to inject a contrast agent into the biliary tract; third, biliary obstruction is relieved by placing a stent, draining bile, and removing stones. ERCP can be combined with EST when necessary to relieve the hypertensive state caused by ampullary obstruction. Since the 1970s, ERCP and EST have become the preferred minimally invasive interventional strategies for treating diseases of the biliary and pancreatic systems [32, 33]. EST, as a combined minimally invasive therapy based on ERCP, plays a crucial role in the treatment of choledocholithiasis, acute obstructive suppurative cholangitis, and biliopancreatic ampullary sphincter of Oddi dysfunction. EST not only provides a timely and effective diagnosis and treatment for patients with biliary obstruction or ABP but can also improve the prognosis and avoid delay in secondary ERCP. Although ERCP or ERCP combined with EST has good clinical efficacy in the treatment of ABP, it may lead to complications such as bleeding, perforation, bile duct infection, and postoperative pancreatitis during diagnosis and treatment because of its invasiveness and potential surgical risks. Studies have shown that the incidence of post-ERCP complications was 9.8%, and 0.4% in patients who died from complications directly or indirectly due to ERCP [34]. Theoretically, using ERCP to dredge the biliary tract can prevent adverse events, and thus for patients with ABP, early ERCP has better efficacy than conservative treatment. However, some RCTs have found that ERCP does not provide such benefits, and there are still many controversies about the benefits of early application of ERCP in the treatment of ABP [35–37]. Therefore, it is still important to explore the clinical efficacy of ERCP or ERCP combined with EST in treating ABP in patients.
In previous literature, some studies compared the effects between ERCP and the conventional approach. For instance, Shrestha et al. investigated the outcome of early ERCP with the conventional approach in ABP patients without acute cholangitis [36]. They performed an online search of PubMed, PubMed Central, Embase, Scopus, and Clinicaltrials.gov databases for relevant studies published until December 15, 2020, and found that early ERCP for ABP without cholangitis was not superior to conservative treatment in terms of mortality, complications, and other adverse outcomes. Coutinho et al. performed a systematic review of 10 RCTs evaluating ERCP versus the conventional approach and reported that early ERCP decreased the risk of local complications, shortened the time to pain relief and hospital stay and was more cost-effective [35]. In 2011, De Lisi et al. also performed a systemic review to evaluate the performance, risk of complication, course of pancreatitis, and hospital stay of EUS compared with ERCP [38]. Based on their literature search and analysis for studies published from 1994 to 2010, they reported that EUS-based treatment in ABP patients was a safer and more effective strategy. Thus, the role of ERCP compared to conventional management has been controversial based on existing literature.
However, considering the advances in the treatment of ABP, existing literature on ERCP might be considered limited because the studies investigated (1) did not involve treatment strategies based on the latest clinical guidelines and the treatment used might have been somehow outdated, (2) focused on ABP without cholangitis or RCTs, (3) was performed in early or urgent clinical settings, (4) considered ERCP alone rather than in combination with other treatments such as EST, and (5) did not consider common clinical observations that are usually made even in less-resourced hospitals such as postoperative abdominal pain, time to intestinal exhaust, serum biochemical parameters and inflammatory factor levels, etc.; Therefore, to a certain extent, they limit their conclusions to the wider population. Thus, taking these limitations in the current literature into consideration, in the present study, we systematically assessed the clinical efficacy of ERCP in patients with ABP irrespective of cholangitis status, using the latest publications of the past decade (January 2010 to January 2022), and included studies with ERCP only or ERCP with other combination treatment as well. In total, 15 studies were included in this meta-analysis, which avoided the disadvantages of the small sample size of single studies and low statistical power. We quantitatively compared the effective rate, incidence of complications, symptom relief, length of hospital stay, serum biochemistry and inflammatory factors between the observation and control groups. Overall, our results indicated that ERCP or ERCP combined with EST was superior to conservative treatment in terms of treatment efficacy and incidence of complications. In addition, ERCP combined with EST was associated with a lesser extent of abdominal pain, faster recovery time of exhaust and serum amylase, and reduced length of hospital stay and levels of Tbil, WBC, CRP, and TNF-α after treatment. Collectively, ERCP had a positive role in guiding the clinical diagnosis and treatment of ABP.
Despite the important observations of this meta-analysis, there were still some limitations that should be clarified. First, some of the studies included were classified as low quality. Second, different criteria for diagnosing the severity of ABP might have existed in the included studies, and different timing of ERCP intervention may have been performed. Third, ERCP is usually performed on an outpatient basis and post-procedurally it can lead to pancreatitis [39]. No post-ERCP follow-up was performed in the included studies. Additionally, only some studies assessed the advantages of ERCP in moderate and severe ABP.
Conclusions
Our results showed that ERCP or ERCP combined with EST was superior to conservative treatment in clinical efficacy, relief of abdominal pain, recovery time of intestinal exhaust and serum amylase, length of hospital stay, and levels of Tbil, WBC, CRP, and TNF-α for ABP. The findings of this study could be used to help guide and standardize the diagnosis and treatment for related diseases. However, considering the limitations of the current literature, more high-quality RCTs with larger sample sizes, multiple center settings, and rigorous designs are still needed to confirm the clinical efficacy of ERCP in these patients.