Introduction
Cutaneous lichen planus (CLP) is a papulosquamous eruption of skin which is characterized by violaceous papules and plaques and mostly located on the extremities but may occur in the other parts of the body or oral and genital mucosa. CLP is a benign dermatological disease, however, patients are often troubled by pruritus and pigmentation. The current first-line therapy is topical corticosteroids [1]. Although treatment with topical corticosteroids is effective, in most trials, quality levels of the body of evidence range from very low to moderate, so more well-designed studies are needed to investigate the efficacy of topical corticosteroids [2]. Adverse effects of topical steroids are well known and include: thinning of skin, infections, striae, systemic absorption, and hypothalamic-pituitary axis suppression. Therefore, a different treatment regimen for CLP that does not rely on corticosteroids may be beneficial.
Topical tacrolimus and pimecrolimus are effective topical calcineurin inhibitors (TCI), which are anti-inflammatory agents that allow treatment of inflammatory dermatoses without the adverse effects of topical corticosteroids. They are already approved for the treatment of atopic dermatitis in patients older than 2 years, but there are many small series and case reports reporting successful use of TCIs in various other skin diseases [3–6]. Double-blind and open studies have shown favourable results with topical tacrolimus and pimecrolimus in mucosal lichen planus [7–10].
Aim
This retrospective study aimed to compare the effectiveness of topical clobetasol propionate 0.05% and topical tacrolimus 0.1% in patients with CLP.
Material and methods
Overall, 50 patients (27 patients in the clobetasol group and 23 patients in the tacrolimus group) who met the inclusion criteria were enrolled to this case series study retrospectively. Patients who were diagnosed as CLP clinically and histopathologically and who were prescribed topical tacrolimus 0.1% or topical clobetasol propionate 0.05% ointment were enrolled in the study and compared.
CLP was histologically confirmed in all patients and diagnosis was made with the presence of a band-like cellular infiltration comprising mainly lymphocytes and signs of basal cell layer degeneration and Civatte bodies. Patients who had already received or were receiving treatment for CLP with anything other than topical tacrolimus 0.1% or topical clobetasol propionate 0.05% were excluded. Patients receiving systemic immunosuppressants or received them in the last year were also excluded. Demographic features, duration of disease, LP subtype, distribution of lesions, thickness and pigmentation scores, visual analogue scale (VAS) scores, laboratory values, adverse effects and all patient’s week 4, 8, and 12 follow-up notes were retrieved from the database.
Lesions were assessed by the patients themselves with regard to pigmentation of lesions and severity of pruritus at every visit using a VAS with a range of 0–10 with higher numbers indicating worse outcomes. At week 12,patients were classified according to the response to the treatment (elimination of the pruritus, elevation and erythema of the lesions), as complete response (more than 90%), partial response (50–90%), weak response (20–50%), and no response (less than 20%).
The study was performed according to the Helsinki declaration and an ethics committee’s permission was obtained (on 07/01/2016, number: 23521).
Statistical analysis
Data analyses were performed using statistical software SPSS 15.0. Descriptive statistics are given as numbers and percentages for categorical variables, and averages and standard deviations for numeric variables. Comparisons between two independent groups were made using Student’s t-test when numeric variables fulfilled the condition of normal distribution, and the Mann-Whitney U test when they were not normally distributed. Comparisons of two dependent groups were made using the paired samples t-test when the differences of variables were normally distributed, and the Wilcoxon test when this criterion was not fulfilled. Under the circumstances where conditions could not be fulfilled, Monte Carlo simulation was applied. The statistical ɑ (level of significance) level was accepted as p< 0.05.
Results
Patients’ demographic and disease characteristics are summarized in Table 1. There were no statistically significant differences between two groups regarding age, sex or disease duration.
Table 1
Baseline demographics and medical information
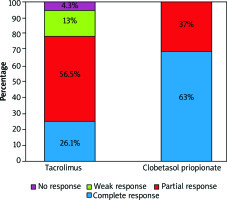
Both groups showed improvement in VAS scores (Figure 1). At week 12, complete response was 63% (n = 17) in the clobetasol group and 26% (n = 6) in the tacrolimus group (Figure 2). Ten patients responded partially in the clobetasol group and 13 patients in the tacrolimus group at the end of treatment. No treatment failure was observed in the clobetasol group.
Figure 1
Patient VAS scores of pruritus (A) and pigmentation (B) by the treatment group. Both the clobetasol group (dashed line) and the tacrolimus group (solid line) showed an improvement
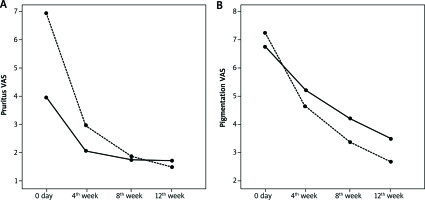
There was a significant decrease in VAS scores regarding pruritus and pigmentation for both groups. However, there was a statistically higher improvement of VAS scores for pruritus and pigmentation found at week 12 with clobetasol (p = 0.004, p = 0.038).
There was no statistically significant difference in terms of adverse cutaneous effects between the two groups. None of the patients in the tacrolimus group reported striae, telangiectasia or local infection. However, burning/pain symptoms were reported by 2 patients in the tacrolimus group and 5 patients in the clobetasol group in the overall treatment.
The mean lymphocyte count in peripheral blood did not decline after treatment in either group and statistical analysis of the counts of lymphocytes in peripheral blood before and after tacrolimus treatment showed no statistically significant difference (p = 0.879).
The mean overall clinical response at week 12 and disease duration showed no significant correlation in either treatment group (p = 0.734, p = 0.999).
Discussion
Recently, topical tacrolimus and pimecrolimus have been shown to be effective in various inflammatory dermatoses without the adverse effects of topical corticosteroids. Oral lichen planus is one of the best studied off-label uses for topical calcineurin inhibitors [9–13]. In one head-to-head study involving 32 patients, tacrolimus 0.1% ointment and clobetasol propionate 0.05% ointment showed significant improvement from baseline, and notably, tacrolimus was significantly better than clobetasol (p< 0.001) [14]. In another study of 30 patients with oral erosive/ulcerative lichen planus, tacrolimus 0.1% ointment was as effective as topical clobetasol propionate 0.05% ointment, both showing significant improvement from baseline [15]. In a retrospective study, topical tacrolimus therapy improved lesions in 15 of 16 women with vulvar lichen planus [16].Nevertheless, in one study, only 7 patients of 21 who were diagnosed with oral lichen planus and treated with topical 0.1% tacrolimus twice daily showed complete response at month 6; although it was a retrospective study, the authors suggested that the efficacy of topical tacrolimus was overestimated in daily practice [17].
Al-Mutairi et al. reported 13 patients with lichen planus pigmentosus who were treated with tacrolimus ointment and 7 (53.8%) of them showed prominent lightening of the pigmentation within 12 weeks [18]. Our patients with pigmented and actinic lichen who were treated with tacrolimus planus also had a favourable response (Figures 3 and 4). Pigmented and actinic lichen planus mostly occurs on light-exposed areas and needs longer treatments more than other forms. Therefore, topical tacrolimus may be preferred before topical corticosteroids for lesions on the face, which is very sensitive to long-term cutaneous adverse effects of topical corticosteroids.
Figure 3
A patient with pigmented lichen planus before and after 12 weeks’ treatment with topical tacrolimus 0.1%
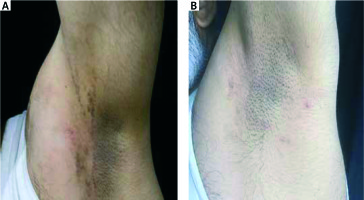
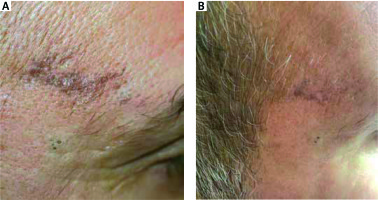
Figure 4
A patient with actinic lichen planus before and after 12 weeks’ treatment with topical tacrolimus 0.1%
A pilot study showed pimecrolimus cream 1% as an effective treatment regimen for severe lichen sclerosus in patients who were unsatisfactorily treated with topical corticosteroids previously [19]. Therefore, a longer treatment period in the tacrolimus group may have been more effective in our patients who showed partial, weak or no response. Also Paola et al. reported a successful outcome with pimecrolimus in hypertrophic genital lichen planus [20].
Tacrolimus has been studied too many times and has an acceptable safety profile. Simon et al. reported a decrease in lymphocyte counts in peripheral blood in10 patients with atopic dermatitis who were treated with topical pimecrolimus [21]. In our study, the mean lymphocyte count in peripheral blood did not decline after treatment in either group.
In the literature, reported adverse effects of TCIs are rare and include mostly itching or burning sensation [22]. Although more patients reported erythema and pruritus in the tacrolimus group, there was no statistical difference between the groups. Interestingly, burning and pain were reported by more patients in the clobetasol group. On the other hand, striae, telangiectasia, and local infections were reported only in the clobetasol group.
Limitations of our study were a small sample size, retrospective design and non-homogeneous distribution of specific LP types to treatment arms and non-usage of life quality indexes.
Conclusions
Both clobetasol and tacrolimus ointments are effective in the treatment of CLP. Our study suggests that topical clobetasol is more effective so it remains the most appropriate first-line therapy for CLP. However tacrolimus can be an option for patients who have failed therapy with topical corticosteroids or those who are contraindicated for the use of corticosteroids. It may be preferred before topical corticosteroids for lesions on the face, neck, and intertriginous regions of the body, which are sensitive to cutaneous adverse effects of topical corticosteroids.