Introduction
Based on the data from the computed tomography (CT) pulmonary nodules (PNs) screening trial [1–5], sub-centimeter PNs (SCPNs) with a diameter ≤ 10 mm are observed in approximately 15% of the screened population, of which 48–56% of the cases occur in patients with lung cancer [6, 7]. These data indicate that a critical step in the management of SCPNs is the ability to distinguish between malignant and benign SCPNs.
Diagnosis of SCPNs is challenging, although many functional imaging techniques such as positron emission tomography (PET), diffusion weighted magnetic resonance imaging (DWI), and contrast-enhanced CT have been developed to differentiate malignant and benign PNs [8–10]. Patients with SCPNs are suggested to undergo CT follow-up at 6–12 months, and then at 18–24 months. However, studies have found an inverse association between long-term follow-up visits and the mental health of the patients [1].
The minimal invasiveness and high precision of CT-guided biopsy have established this technique as the preferred method for the diagnosis of pulmonary diseases [11–18]. The technical success, diagnostic precision, and complications were usually influenced by the types of needles (core or fine needles), CT guidance (CT fluoroscopy, conventional CT, or cone-beam CT), and lesion size [19], yet further studies are needed to better understand the relationships between these factors.
Aim
We aimed to assess the safety and diagnostic precision of CT-guided core needle biopsy (CNB) for SCPNs.
Material and methods
The Institutional Review Board approved this single-center retrospective analysis. The requirement for written informed consent was waived.
Study design
Between January 2016 and December 2018, consecutive patients with SCPNs (Group A) underwent CT-guided CNB. The inclusion criteria were: (a) a definite CT-based diagnosis of PN; (b) PN ≤ 10 mm; (c) clinic-radiological assessment indicating the presence of PNs with an intermediate to high risk of lung cancer [1]. Patients were excluded based on the following criteria: (a) PN < 5 mm; (b) a reduction in the size of the PN; (c) no change in size of the PN for 2 years; and (d) repeat biopsy for the same PN.
During the same period, patients underwent CT-guided CNB for PNs with the diameter of 11–20 mm (Group B). The baseline data, diagnostic performance, and complication rates were compared between the 2 groups.
CNB procedure
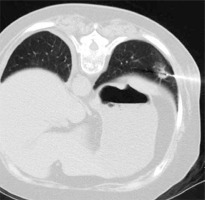
The CT-guided CNB was performed under local anesthesia with a 16-row CT device (Philips, Amsterdam, Netherlands) by an experienced chest radiologist. The needle pathway was decided according to the location of the target PN under the scanning thickness of 1 mm. Initially, the lung parenchyma was perforated using an 18G semi-automatic core needle (Wego, Weihai, China), followed by a CT scan to determine the needle location and to guide further movement. Next, the accuracy of the needle position was verified by establishing a multi-planar reformation of the punctured image (Figure 1). Finally, the samples were collected when the needle tip came in contact with the target lesion. The samples were kept in 10% formaldehyde until further analysis. The presence of any possible CNB-related complications was assessed through a repeat CT scan.
Diagnostic performance
The biopsy specimens were diagnosed as follows: (1) specific benign (benign tumor and bacterial/fungal inflammations), (2) suspected malignant, (3) malignant, and (4) non-malignant.
Final malignant diagnoses were achieved in the following ways: (a) resection; and (b) CNB-based malignant diagnoses were directly used as the final diagnoses.
Final benign diagnoses were achieved in the following ways: (a) resection; (b) CNB-based specific benign diagnoses were directly used as the final diagnoses [10]; and (c) if the lesion size decreased by ≥ 20% or remained stable for 2 years without any anticancer treatment, the final diagnosis would be a benign lesion.
Definitions
The technical success of CT-guided CNB was defined as obtaining a sample of adequate quality to permit visual inspection [7]. The diagnostic yield was determined by dividing the CT-guided CNB based diagnostic results by the total results [7]. Diagnostic accuracy was determined based on the sum of the number of true positive and true negative results [7].
Statistical analysis
All data were analyzed using SPSS v16.0 (SPSS, Chicago, IL, USA). The continuous variables were analyzed using a t-test and the categorical data were analyzed using χ2 tests. Univariate and multivariate logistic regression analyses were used to assess the predictors of diagnostic accuracy. The multivariate analysis included covariates with a p-value < 0.1 in the univariate analysis. A p-value < 0.05 indicated a statistically significant difference.
Results
Patients
The baseline data of the patients in the 2 groups are shown in Table I. Except for the PN size (9.1 ±1.2 mm vs. 16.6 ±3.6 mm, p < 0.001), all of the data were not significantly different between the 2 groups. Twenty-six and 23 patients in groups A and B had a malignant history, respectively (p = 0.360).
Table I
Baseline data between the 2 groups
Technical success
The technical success rates in groups A and B were both 100%. No significant differences were observed in the lesion-pleura distance, needle-pleura angle, patients’ position, number of samples, and duration of the procedures between the 2 groups. In group A, there were 34, 42, and 29 patients who had 1, 2, and ≥ 3 puncture pathways, respectively. In group B, there were 45, 50, and 22 patients who had 1, 2, and ≥ 3 puncture pathways, respectively. There was no significant difference (p = 0.280).
CNB-based diagnoses
In group A, the CNB-based diagnostic results included 41 malignancies, 5 specific benignities, and 59 non-specific benignities. In group B, the CNB-based diagnostic results included 61 malignancies, 3 suspected malignancies, 6 specific benignities, and 47 non-specific benignities. No significant difference was observed in the distribution of CNB-based diagnostic results between the 2 groups (p = 0.052, Table II).
Table II
Biopsy and final diagnoses in the 2 groups
Final diagnoses
Final diagnoses were obtained from all patients (Table II). In group A, CNB-based malignancies (n = 41) and specific benignities (n = 5) could be determined as the final diagnoses. In the 59 non-specific benignities, 48 PNs were verified as benignities based on the CT follow-up (n = 43) or underwent surgical resection (n = 5). The remaining 11 PNs were classified as malignancies based on repeated biopsy (n = 5) or surgical resection (n = 6).
In group B, CNB-based malignancies (n = 61) and specific benignities (n = 6) could be determined as the final diagnoses. The 3 CNB-based suspected malignancies were identified as malignancies after surgical resection. Among the 47 non-specific benignities, 40 PNs were verified as benignities based on the CT follow-up (n = 36) or underwent surgical resection (n = 4). The remaining 7 PNs were classified as malignancies based on repeated biopsy (n = 6) or surgical resection (n = 1). No significant difference was found in the distribution of final diagnostic results between the 2 groups (p = 0.095, Table II).
Diagnostic performance
The diagnostic performance-related data are shown in Table II. There were no significant differences in the diagnostic yield (43.8% vs. 54.7%, p = 0.105), overall accuracy (89.5% vs. 94.0%, p = 0.221), and sensitivity (78.8% vs. 90.1%, p = 0.080) between the 2 groups.
The results of univariate logistic regression analysis showed that the risk factors associated with diagnostic failure of SCPNs included female sex (p = 0.071), number of tissue samples of 1 (p = 0.036), and CNB-related pneumothorax (p = 0.001). When these factors were combined in the multivariate model, the independent risk factor related to diagnostic failure was CNB-related pneumothorax (p = 0.001).
Complications
The CNB-related complication data are shown in Table III. There were no significant differences in the rates of pneumothorax (13.3% vs. 15.4%, p = 0.664) and pulmonary hemorrhage (10.5% vs. 8.5%, p = 0.624) between the 2 groups.
Table III
Details of the procedures
Univariate logistic regression analysis revealed that the risk factors associated with pneumothorax for the patients with SCPNs included decubitus position (p = 0.006) and a high number of needle pathways (p = 0.002). When these factors were combined in the multivariate model, the risk factors related to pneumothorax remained as the decubitus position (p = 0.009) and a high number of needle pathways (p = 0.004).
Univariate logistic regression analysis found that the only risk factor associated with pulmonary hemorrhage for the patients with SCPNs was greater lesion-pleura distance (p = 0.048).
Discussion
In this study, we examined the diagnostic precision and the feasibility of CT-guided CNB for SCPNs. Our results showed that this approach had a 100% success rate and was consistent with the results of previous studies (99–100%) [6, 7]. The main factors associated with the high technical success rate included: (a) a scanning thickness of 1 mm, which helped to find the most appropriate puncture pathway; (b) the multi-planar reformation of the perforated image that helped to adjust the needle from multi-aspects [7].
Our method had a diagnostic accuracy of 89.5%, with a specificity of 100% and sensitivity of 78.8%. Previous studies on CT-guided CNB for SCPNs demonstrated overall diagnostic accuracies in the range 90–98% [6, 7]. Choo et al. [6] achieved a 98% diagnostic accuracy using the C-arm cone-beam CT-guided approach. A meta-analysis also revealed that compared to normal CT-guided lung biopsy, real-time monitoring CT could yield higher diagnostic accuracy [19]. However, the disadvantages of real-time monitoring include unwanted exposure of the operators to radiation.
The sensitivity of 78.8% in this study demonstrated that CT-guided CNB still had false-negative conditions. This sensitivity was slightly lower than previously reported (82–95%) in meta-analyses of quantitative imaging approaches, including PET/CT, contrast-enhanced CT, and DWI [8–10]. However, 100% specificity was achievable, indicating that CNB did not produce false-positive results. Also, CT-guided CNB could account for approximately 43.8% of the definite diagnostic rate.
In this study, the independent risk factor associated with failure to diagnose was CNB-related pneumothorax. The CNB-related complications usually disturb the CNB procedure. Although the quantity of the obtained samples was not related to the diagnostic failure, CNB-related pneumothorax can reduce the quality of the obtained samples.
The regulation of CNB-based non-specific benignities presents a challenge, and the negative prognostic potential of CNB of non-specific benignity was found to be in the range of 84–89% [20–22]. These conditions require regular CT follow-up. However, if the patients are at high risk of false negatives (such as a history of malignancy, metastasis, abnormal levels of tumor markers, or enlarged lesions), then surgical resection of the repeated CNB should be advised.
The rate of pulmonary hemorrhage was 10.5% and the rate of pneumothorax was 13.3%. These data were consistent with the rates reported in previous studies of CT- or C-arm cone-beam CT-guided biopsy for SCPNs [6, 7]. Among these cases with CNB-related complications, most of the patients (23/25, 92%) only received conservative treatment. It indicated the high safety of CT-guided CNB for SCPNs.
Decubitus position, a high number of needle pathways, and greater lesion-pleura distance were risk factors for CNB-related complications in this present study. These risk factors have also been identified in previous studies regarding CT-guided lung biopsy [7, 15]. To decrease the risk of complications, accurate preoperative design of the needle pathway and CT multi-planar reformation technique are required when performing CT-guided lung biopsy.
In this study, the CNB-related data were compared between patients with SCPNs and larger PNs (11–20 mm). CT-guided CNB is a highly accurate and safe method for the diagnosis of PNs ≤ 20 mm with the mean diagnostic accuracy of 90%, pneumothorax rate of 19%, and pulmonary hemorrhage rate of 12% [23]. We found that the diagnostic yield, diagnostic accuracy, pneumothorax rates, and pulmonary hemorrhage rates were not significantly different between the 2 groups. Our results showed that smaller PN might not reduce the diagnostic ability and safety of CT-guided CNB for PNs. A recent study of CT-guided CNB for SCPNs also demonstrated that the size of the SCPN did not impact the diagnostic accuracy and complication rates [24].
This study had several limitations: Firstly, the retrospective nature of this study implies selection bias. Secondly, the absence of a control group restricts its comparison to other techniques for SCPNs (such as C-arm cone-beam CT or CT fluoroscopy). Finally, this study was conducted at a single center and requires further validation in randomized controlled trials.