Introduction
Demodicosis is a skin disease caused by Demodex mites (DM). The two species pathogenic for humans are Demodex folliculorum and Demodex brevis [1]. Demodex folliculorum was first described in 1842 by Simon [2]. DM are known as a part of the normal skin fauna and the most frequent ectoparasites of humans which occur worldwide [2, 3]. The presence of DM in most people is of no consequence [1]. They spread by skin-to-skin contact and the incidence of infestation is highly related to the age of the patient [2, 3]. The disease is mostly acquired at a young age [1]. The pervasiveness of DM in people over 71 years is about 95%. Moreover, men are predominantly more heavily infested than women [3].
Demodicosis is associated with involvement of pilosebaceous units predominantly found in the follicles of the eyelids, the nose and the nasolabial folds [1, 2]. We can distinguish two clinical variants: primary and secondary. Primary demodicosis is related with abnormal escalation in mite colonization in patients with the absence of inflammatory dermatoses. Moreover, remission of the disease occurs only after the proper treatment [1]. Primary demodicosis is associated with pityriasis folliculorum, nodulocystic demodicosis, blepharitis, perioral dermatitis and auricular demodicosis [1, 2].
Secondary demodicosis is classified as the presence of DM in patients with other skin or systemic diseases. The disease is frequently recognized in immunosuppressed patients [1]. A more extensive variety of manifestations of demodicosis are found in immunosuppressed patients compared with immunocompetent people [3]. However, there can be found limited literature data based on major groups confirming those assumptions (Table 1). Most often the authors present only single cases of patients with demodicosis under immunosuppression due to organ transplantation, HIV/AIDS and others (Table 2).
Table 1
Studies on demodicosis including patients under immunosuppression
| Year of publication | Authors of the study | Number of patients in the study | Reason for immunosuppression |
|---|---|---|---|
| 1998 | Roihu et al. [39] | 40 | DLE |
| 2001 | Aydingöz et al. [11] | 12 | Organ transplantation |
| 2007 | Ciftci et al. [46] | 41 | Rheumatoid arthritis |
| 2008 | Kulac et al. [34] | 45 | Phototherapy |
| 2011 | Gerber et al. [44] | 19 | EGFR inhibitors therapy in cancer |
| 2012 | Teraki et al. [37] | 16 | Tacrolimus ointment |
| 22 | Steroid | ||
| 2013 | Kosik-Bogacka et al. [45] | 95 | Haematological diseases (acute leukaemias, non-Hodgkin’s lymphomas, chronic lymphocytic leukaemia, myeloproliferative syndromes, multiple myeloma) |
| 2013 | Wiwanitkit et al. [19] | 60 | HIV |
| 2015 | Talghini et al. [40] | 32 | DLE |
| 2019 | Arli et al. [41] | 30 | Allergic rhinitis |
| 30 | Diabetes | ||
| 32 | Both allergic rhinitis and diabetes | ||
| 2020 | Dursun et al. [38] | 42 | DLE |
| 2020 | Keles et al. [4] | 45 | Psoriasis vulgaris, pemphigus vulgaris, alopecia areata, or lichen planus |
| 2020 | Yüksel et al. [42] | 36 | Heart failure |
Table 2
Case reports presenting patients under immunosuppression with demodicosis
| Year of publication | Authors | Number of patients with demodicosis | Reason for immunosuppression or immunosuppressive treatment | Presented symptoms | Treatment |
|---|---|---|---|---|---|
| 1989 | Ashack et al. [31] | 1 | HIV | Folliculitis, pruritus | Lindane |
| 1989 | Dominey et al. [26] | 2 | HIV | Papular, nodular, vesicular, eruption, pruritus | Benzene hydrochloride/ Permethrin |
| 1991 | Banuls et al. [23] | 1 | HIV | Papular eruption, pruritus | Crotamiton |
| 1992 | Sanchez-Viera et al. [24] | 1 | HIV | Papular, pustular eruption | Erythromycin |
| 1993 | De Jaureguiberry et al. [25] | 1 | HIV | Papular nodular, pustular eruption, pruritus | Prioderm |
| 1993 | Redondo Mateo et al. [22] | 1 | HIV | Papular, pustular eruption, pruritus | Crotamiton |
| 1996 | Barrio et al. [27] | 1 | HIV | Papules | Erythromycin and metronidazole |
| 1998 | Sarro et al. [28] | 1 | HIV | Area of dry skin | Topical sulphur |
| 1999 | Patrizi et al. [29] | 1 | HIV | Papules, pustules | Crotamiton |
| 2001 | Jansen et al. [30] | 1 | HIV | Papules, pustules | Permethrin |
| 2002 | Aquilina et al. [21] | 1 | HIV | Papular and pustular eruption | Ivermectin and permethrin |
| 2003 | Lübbe et al. [36] | 1 | Pimecrolimus cream | Papular facial flare | Doxycycline |
| 2004 | Delfos et al. [33] | 2 | HIV | Maculopapular and pustular rash | Metronidazole/ivermectin |
| 2004 | Antille et al. [35] | 1 | Tacrolimus ointment | Rosacea | Doxycycline |
| 2006 | Lotze et al. [14] | 1 | Chronic idiopathic myelofibrosis | Rash mimicking aGvHD | Lindane |
| 2008 | Aisa et al. [15] | 2 | Leukaemia | Multiple papules and pustules with erythema mimicking aGvHD | Topical sulfur |
| 2012 | Roman-Curto et al. [16] | 1 | Leukaemia | Facial erythema with oedematous micropapules mimicking aGvHD | Permethrin and metronidazole |
| 2013 | Cotliar et al. [17] | 1 | Leukaemia | Patchy and confluent erythema | Ivermectin |
| 2014 | Yamaoka et al. [20] | 1 | HIV | Butterfly rash-like rosacea | Crotamiton |
| 2016 | Chovatiya et al. [9] | 4 | Organ transplantation | Acneiform eruption, papules | Doxycycline/permethrin |
| 2018 | Chen et al. [13] | 2 | Leukaemia/primary myelofibrosis | Monomorphic erythematous papules with oedema mimicking aGvHD | Ivermectin and permethrin |
| 2019 | Hachfi et al. [32] | 1 | HIV | Maculopapular, pustular and squamous erythematous rash | Metronidazole |
| 2020 | Tahir et al. [12] | 1 | leukaemia | Facial puffiness, redness and bilateral periorbital oedema | Ivermectin |
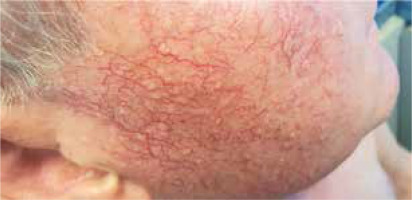
A diagnosis of demodicosis is made on the basis of microscope analysis of scrapings of the skin or standardized skin surface biopsies (SSSB) from different regions like the forehead, cheeks, nose or chin. Determination of five or more parasites in 1 cm2 area using a light microscope is considered as a positive result [1, 4]. Symptomatic demodicosis may be observed as acne rosacea, pityriasis folliculorum, papulopustular eruptions, perioral dermatitis or blepharitis (Figure 1).
Treatment of demodicosis is weakly evidence based. In the therapy, mostly used topical sulfur products are: permethrin and ivermectin [1, 3]. Facial Demodex infestations are also susceptible to doxycycline, erythromycin, dilute topical camphor and metronidazole [1, 3].
To this date it has not been clear which groups of patients are clearly prone to develop demodicosis. The conditions and medications affecting humoral and cellular immunity might cause the proliferation of DM. The literature predominately mentions immunosuppressive treatment, HIV/AIDS, cancer and diabetes.
Aim
This article presents collected knowledge focusing on groups of patients under immunosuppression.
Methods
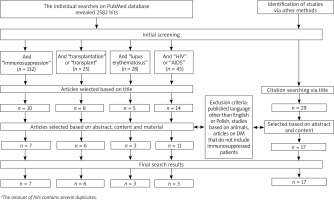
The search was conducted following the PRISMA guidelines by two individual reviewers. The last date of investigation was 1 March 2021. The search aimed to find data on DM among immunosuppressed patients. Search terms included (demodicosis OR demodex) AND (immunosuppression OR transplant OR transplantation OR HIV OR AIDS OR lupus erythematosus). PubMed was the main search engine. We excluded studies concerning animals. A few articles were excluded or included only based on abstracts due to the language other than English or Polish. Additional articles were identified through reference search of the articles acquired via PubMed. The selection process resulted in a total amount of 13 original works, 23 case reports and 10 review articles on immunology, pathophysiology, treatment or other aspects of demodicosis. Details of the selection process are presented in Figure 2.
Pathogenesis
The pathogenesis of demodicosis is not entirely explained. A critical point is a transition to an inflammatory stage. It is largely obscure whether demodicosis is caused by a huge amount of DM or by an overshooting immune response. Some authors estimate that the identification of cathelicidin LL-37 in inflammatory dermatoses and the variance expression of various cytokines or proteins involved in inflammasome activation indicated the interaction between skin congenital immunity and microbial homoeostasis [1, 5].
Demodex mites induce a strong immune response. Follicles infested with DM are sometimes surrounded by spongiosis and lymphatic infiltrates. Tissues from rosacea-like patients have upregulation of inflammatory cytokines: tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β) and interleukin 8 (IL-8) [1]. The immune response is connected with the HLA- type. The HLA Cw2 phenotype increases the risk of infestation. Contrarily the HLA A2 phenotype seems to be protective. Patients without this allele recruit fewer CD8+ lymphocytes, have a less functional leukocyte response and might develop a humoral response with higher IgA concentrations [3]. This group is at risk of developing deep papular and papulopustular lesions that occupy larger areas of the skin [6].
Akilov and Mumcuoglu presented the immune response based on 29 patients with demodicosis. It was evaluated in the peripheral blood by identifying membrane markers of immune cells [7]. As shown, the absolute number of CD95+ was higher, whereas numbers of CD3+, CD4+, CD8+, CD16+ and the activity of leucocytes were lower. The number of CD20+ and function of B cells seems to be similar as in the control group. T cells seem to be the target of immunosuppression in DM infestation [8]. Apoptosis is a basic mechanism for positive and negative selection of T cells and B cells, which is important in the elimination of defective cells with ability to autorespond (respond against itself). However, a defect in the control system of apoptosis may lead to complications in the immune system, what can be the leading problem in demodicosis, as CD95+ cells are 2.5 times increased [7].
Demodicosis in patients after organ transplantation
Organ transplantation is in many cases a life-saving or life-extending procedure. However, patients after solid organ transplantation are prone to undergo many diseases as a consequence of immunosuppression. Skin infections that may be diagnosed more often are for example: herpes simplex zoster, varicella zoster virus or gram-positive bacteria. There are also a few articles connecting organ transplant recipients with demodicosis. In a case report presented by Chovatiya and Colegio we can find 4 cases of patients after renal transplantation with confirmed demodicosis [9]. A 66-year-old woman 4.5 years after renal transplant was referred to a transplant dermatology clinic by a nephrologist because of acute acneiform eruption. Microscopic evaluation revealed numerous DM. Then, a 55-year-old woman 6 months after transplantation, who also presented with pruritic acneiform papules, was positive for DM by mineral oil preparation. The third case presents a 44-year-old man after a second renal transplant 2 years earlier, after the first transplant in 1994. Leading symptoms of demodicosis were facial pustules. In the last case a 55-year-old man, 2 years after renal transplantation and 14 years following bone marrow transplantation, was referred to the clinic because of waxing and waning acneiform facial eruption on the forehead and cheeks. The patient was also diagnosed with demodicosis. All of the presented patients had previously unremarkable dermatological histories. The time between transplantation and the presentation of demodicosis varied from 5 months to 5 years. Interestingly, only 1 of 4 patients responded with full resolution to 5% permethrin. The other three resolved on oral antibacterial medication (doxycycline) within 1 year. The authors suggest that the problem of demodicosis as a secondary disease to solid organ transplantation may be more common than previously thought [9].
In the article by Aydingöz et al. in 1997, no correlation between immunosuppression and DM density was found based on two samples of SSSBs in a group of 30 organ transplant recipients [10]. The study was in 2001 revisited due to recommendations on the SSSB method modification. However, the change of method did not change the results of the study and diagnosis of 12 organ transplant recipients did not show any correlation between immunosuppressive therapy and organ transplantation [11]. Authors suggest that there may be factors other than immunosuppression influencing DM density. However, it must be highlighted that the group contained thirty, and then in 2001 only twelve renal transplant recipients. It is clear that bigger studies in this population are required as there is no more information to be found about demodicosis among patients after solid organ transplantation.
Similarly to solid organ transplantation groups, the literature presents only few case reports among patients after stem cells transplantation (SCT). In those cases, demodicosis mimics an acute cutaneous graft versus host disease (aGvHD). aGvHD is the main cause of facial erythema in this group of patients, but other reasons for the lesion must be also considered, for example drug reaction, viral exanthema or infestation of DM. Tahir et al. presents a 73-year-old patient after allotransplantation taking cyclophosphamide and cyclosporine [12]. After 32 days, a sudden onset of facial puffiness, redness and bilateral periorbital oedema was reported. Because of the concerns of aGvHD, topical hydrocortisone, oral antihistamines and systemic corticosteroids were recommended. As the symptoms did not resolve, biopsy was taken, what revealed several DM. The rash responded to two doses of ivermectin [12]. Chen et al. in another case report described 2 patients [13]. After about a month of immunosuppression therapy after SCT, erythematous papules with follicular prominence were reported. The differential diagnosis led to taking facial scrapings of both patients what revealed demodicosis. Both patients’ eruptions resolved after treatment with ivermectin and permethrin [13]. The authors stated that to that point (2018) there were only 5 reported cases of demodicosis after SCT that were mistaken with aGvHD [14–17]. The time between transplantation and the onset of facial erythema varied from 23 to 197 days. What can be interesting about those cases, is the sharp border between the scalp and forehead. The “cut off” sign in demodicosis can be an important diagnostic tool and may help distinguish it from cutaneous aGvHD [12, 13].
Demodicosis in HIV patients
The human immunodeficiency virus (HIV) is a globally occurring pathogen that over time leads to the acquired immunodeficiency syndrome (AIDS). Progressing failure of the immune system, in the form of low levels of CD4+ T cells, dendritic cells and macrophages, may lead to several opportunistic infections and cancers. It has been frequently observed that among adults with HIV, there is a higher chance of papulopustular-type rosacea. Skin symptoms may be an important issue when considering HIV/AIDS as in many cases it can first manifest this way [18]. In the survey by Somsri and Wiwanitkit, among 60 patients with HIV, the rates of eyelash Demodex were 95% for patients with CD4+ count under 200 cells/ml, 70% for patients with CD4+ count between 200 and 500 cells/ml and finally 20% for those with over 500 CD4+ cells/ml [19]. As presented, there can be a strong correlation between the number of DM and CD4+ count. Due to the lack of studies carried out among bigger groups of patients, below we present cases that show the importance of considering Demodicosis as a skin manifestation of HIV/AIDS.
The case report presented by Yamaoka et al. highlights the observation of skin symptoms as very important when considering first manifestations of HIV [20]. In the face-skin biopsy of a patient who was at first suspected to have lupus erythematosus, there were found many DM and a variety of cellular infiltrates. What is more, in immunofluorescence a reduction of CD4+ was revealed whereas the number of CD8+ was elevated. After precise investigation of the patient’s history which confirmed multiple sex partners, a serological test was taken. The CD4+ count was decreased to 62 cells/ml and his HIV viral load was 2400/ml. As authors report, approximately 90% of HIV patients suffer from skin or mucous membrane lesions [20].
In the case report and literature review by Aquilina et al., a patient who was diagnosed with AIDS (stage 1) 1 year before, developed symptomatic demodicosis. The onset of symptoms was about 2 months after the beginning of antiretroviral therapy [21]. The CD4+ count was 150 cells/ml and HIV RNA was 200,000 copies/ml. After 1 month of therapy, the patient had a rapid virologic response with an undetectable viral load and an increase in CD4+ to 210/ml. After 2 months, the patient developed facial eruptions as papules and papulopustular forming plaques with erythema and oedema. The patient had no history of any facial features such as rosacea. After no response to ketoconazole and metronidazole, the scrapings of the face were taken and it demonstrated numerous DM. After treatment with ivermectin and 5% permethrin, no recurrence was observed within 1 year. What is remarkable about this case is that symptoms and inflammatory processes began while the immunity of the patient was being restored. As far as we are concerned, there are more cases in which patients with HIV/AIDS start to present demodicosis symptoms after the beginning of highly active antiretroviral therapy [22–31]. Interestingly, the case report presented by Hachfi et al., found another suggestion on the topic [32]. The author presents a 34-year-old patient suffering from maculopapular and pustular rash on the face and upper limbs. Examination of scrapings enabled the identification of DM. What is more, leucolymphopenia and thrombocytopenia suggested an underlying immunosuppression, leading to the diagnosis of HIV (CD4+ 81 cells/ml, HIV load 70 400 copies/ml). Despite the anti-parasite treatment, 10 days after the beginning of antiretroviral treatment worsening of skin condition was observed. Inflammation and rapidly decreasing HIV load suggested the presence of immune reconstitution inflammatory syndrome (IRIS). Corticosteroid therapy was necessary for 6 weeks. Similar observation can be seen in 2 cases presented by Delfos et al. [33]. The authors hypothesized that an aggravation of symptoms is caused by IRIS and thus by a local influx of immune-associated T cells. It seems that before the onset of anti-retroviral treatment, the immune response is not strong enough to cause visible lesions. However, the delay between onset of anti-parasite and anti-retroviral treatment, to prevent the occurrence of IRIS, has not been yet determined [33].
Demodicosis in other conditions and diseases associated with immunosuppression
Demodicosis as a skin disease is also found in other conditions and diseases associated with immunosuppression.
One of the most common cases is a presence of Demodex folliculorum in patients receiving phototherapy. Ultraviolet light (UV) is used in several dermatological diseases. Its immunosuppressive character helps to decrease symptoms in inflammatory diseases such as atopic dermatitis or psoriasis. However, it is considered to indicate the growth of DM as was presented in Kulac et al. study [34]. Forty-five patients (age range: 13–63), who received phototherapy based on a specific protocol suitable for each individual, were described. Thirteen, as compare to only three in the control group, were diagnosed with demodicosis. Clinical symptoms were present in eight of those thirteen patients. What can be also seen is that patients receiving PUVA were more likely to develop demodicosis than patients receiving narrow-band-UVB (7 out of 12 patients with PUVA compared to 6 out of 33 patients with narrow-band-UVB) what can be explained by the fact that UVA penetrates deeper in tissues and so causes a deeper immunosuppressive effect than narrow-band-UVB [34].
Moreover, DM could be connected with local immunosuppression therapy. Tacrolimus and pimecrolimus are commonly used in inflammatory skin diseases such as atopic dermatitis (AD). Their mechanism includes inhibition of T-cell activation. Antille et al. presented a patient suffering from AD [35]. After several months of tacrolimus ointment treatment of the face, the patient developed rosacea. What is more, a similar case was reported 1 year earlier by Lübbe et al. [36]. A patient with AD suffered from papular facial flare with abundant DM after pimecrolimus 1% ointment use for a few days. Both cases responded to doxycycline therapy. Then, in 2012, a bigger study was presented by Teraki et al. [37]. Forty-four patients with rosacea-like dermatitis were retrospectively reviewed, including those caused by tacrolimus and/or steroid ointments. DM were detected in higher density in 4 out of 7 patients with tacrolimus induced rosacea-like dermatitis and in 5 out of 6 patients with steroid induced rosacea-like dermatitis [37].
Some authors estimate that the frequency of demodicosis in discoid lupus erythematosus (DLE) patients is higher than in the immunocompetent population. The correlation between DM and DLE may differ in different populations, as there can be found opposite articles on the topic. Dursun et al. in his project evaluated retrospectively the files of 42 patients with DLE. 50% of patients were Demodex positive [38]. In addition, the percentage of neutrophils was higher in the Demodex positive patients and the intensity of DM correlated positively with that. On the other hand, in the literature there can be also found studies denying connection between DLE and demodicosis. Roihu and Kariniemi studied the prevalence of DM in facial skin biopsies from a total number of 160 patients (80 patients with rosacea, 40 with facial eczematous eruption and 40 with DLE) [39]. The density of DM was significantly higher in the rosacea group than in eczema or DLE. Also, Talghini et al. in their study compared associations between demodicosis and basal cell carcinoma (BCC), squamous cell carcinoma (SCC), melanoma, DLE, and rosacea in 144 patients [40]. DM infestation rate did not differ significantly between controls and BCC, SCC or DLE, whereas it was higher in rosacea. Interestingly, the DM density was lower in melanoma. In both Roihu et al. and Talghini et al. works, immunosuppressive therapy does not seem to correlate with demodicosis [39, 40].
DM might be also connected with allergic rhinitis (AR) and diabetes mellitus. AR affects approximately 20–40% of the global population causing symptoms such as itching of the nose, discharge, sneezing, obstruction and itching and watering eyes. Diabetes increases the tendency for infection and may be the reason for development of complications in the long term. The study was conducted in 2014–2017 at the Ear, Nose and Throat (ENT) and Endocrinology Polyclinics of Mustafa Kemal University Medical Faculty Hospital, Turkey [41]. It included 92 patients, aged 18–70 years. The patients were divided into 3 groups: 30 diagnosed with AR, 30 diagnosed with Diabetes and 32 with both AR + Diabetes. DM positivity was determined in 44 (47.8%) of all patients and in 1 (3.3%) of the 30 subjects from the control group. In the patient group, DM was present in 14 (43.7%) Diabetes patients, in 12 (40%) AR patients and in 18 (60%) AR + Diabetes patients [41].
The last but not less important group is patients with heart failure (HF). In the study conducted by Yüksel et al., the presence of DM was evaluated in 36 hospitalized HF patients compared to 36 healthy controls [42]. At least one DM was detected in 20 (56%) HF patients and in 9 (25%) people of the control group, whereas demodicosis was positive in 14 (40%) HF patients as 5 DM in 1 cm2 are sufficient to confirm the diagnosis. The suggested reason for it is that both inflammation and immune system dysfunction are considered serious HF progression factors [42].
Discussion
Through the years, pathogenic features of DM have been confirmed and denied. Considering the percentage of the population that carry the commensals without any symptoms it is sure that there must be factors that allow proliferation of mites to the critical level. Usually, the host’s immune system tolerates the presence of mites and keeps its number under control [43]. The border between DM considered as commensals and then as parasites is discussed in many articles. The growing number of DM initiates the humoral immune inflammatory response causing visible cutaneous changes. Many possible factors have been called. A study showed that patients with papulopustular rosacea have an increased pH of the face and the hydration level is reduced [43]. Gerber et al. in their work demonstrates that DM number in patients with papulopustular lesions is induced by the epidermal growth factor receptor (EGFR) inhibitor [44]. This strengthens the role of immunosuppression in the facilitation of mite proliferation. Keles et al. checked the density of DM at the beginning of immunosuppression therapy of 45 individuals [4]. The tests were made before the therapy, 1 month and 3 months into therapy. The test was negative at the beginning in all of the patients and then positive in 1 and 3 patients, 1 and 3 months later, respectively. In the control group 1 person was positive in the whole study. The results suggest an association between immunosuppressive therapy and the number of DM. However, we can also find studies denying the hypothesis. Kosik-Bogacka et al. compared the proportion of DM in the eyelashes of healthy and immunocompromised patients [45]. Eyelashes were taken from 95 patients and 1091 controls. In the study, immunosuppression did not seem to increase the rate of blepharitis as proportions and symptoms were similar in both groups. Unfortunately, no similar study on skin demodicosis was found to compare with ocular demodicosis. Ciftci et al. investigated 41 patients with RA and 27 matched controls. Similarly, the authors found no correlation between patients with RA and demodicosis [46].
The biggest limitation of this article is that studies with opposite results and conclusions can be found. Due to the fact that immunosuppressed patients are a varied and heterogeneous group, it may be hard or impossible to draw one, strong conclusion. The correlation between demodicosis and immunosuppression probably depends on the duration of immunosuppression, level of immunosuppression or skin type.
Conclusions
It is confirmed that demodicosis occurs under various clinical manifestations. Immunosuppression may be considered as a factor increasing the risk of this infection. However, this establishment is based mostly on case reports and limited groups, so bigger studies among immunosuppressed patients are required.
It is probably important to take under consideration the occurrence of demodicosis during the diagnostic process of single or multiple skin lesions on the face in the medical examination of patients under immunosuppression. Especially that the test is inexpensive and not particularly difficult. It leads to starting of the appropriate treatment and improvement of the condition of the skin.