Demodicosis is a disease of the pilosebaceous unit, involving mainly face and head areas, caused by Demodex mites (Demodex folliculorum and Demodex brevis) [1]. These mites can be found in normal skin biopsies and are considered pathogenic if the density measured in standardized skin surface biopsy exceeds 5 mites per cm2 of the skin [1, 2]. Demodicosis can manifest as chronic inflammatory eruption resembling bacterial folliculitis, rosacea, perioral dermatitis, blepharitis and otitis externa [3]. In 2014, Chen and Plewig [1] proposed a new classification dividing human demodicosis into a primary and a secondary form. Secondary demodicosis occurs most commonly in immunosuppressed patients. In this group demodicosis symptoms appear most likely on the face and eyelids, but they can also manifest on other areas of the body. We would like to present a report on demodicosis in a patient with Crohn’s disease treated with an anti-TNF-α medication, infliximab.
A 27-year-old male presented in February 2019 to the dermatology outpatient department with a 6-month history of a red plaque on the left cheek. It started 3 months into the treatment with infliximab, introduced for an exacerbation of Crohn’s disease. According to the patient, it resembled a pustule on an erythematous base but gradually increased in size. On examination there was an infiltrated, erythematous and scaly plaque on the left cheek (Figure 1 A) and a much smaller one on the right one. Initially, a 2-month course of lymecycline (300 mg, once daily) was prescribed with no clinical improvement, therefore, a decision to take an incisional biopsy was made. Histopathological examination showed numerous Demodex mites within the enlarged hair follicles (Figure 2). Reactive lymphohistiocytic infiltrate was present under the epidermis, around hair follicles and blood vessels. The diagnosis of demodicosis was made and the treatment with oral metronidazole (250 mg, 3 times daily) for 2 weeks followed by topical metronidazole (1%, gel, once daily) for 1 month and topical ivermectin (1%, cream, once daily) for 3 months was prescribed. Over the following weeks the patient’s skin condition improved until the lesions completely subsided (Figure 1 B). During follow-up visits in September 2019 and 12 months later, the skin of the left cheek was smooth, with some postinflammatory hyperpigmentation and a small scar present at the biopsy site.
Figure 1
Clinical image of a male with demodicosis on the left cheek before treatment (A) and after treatment (B)
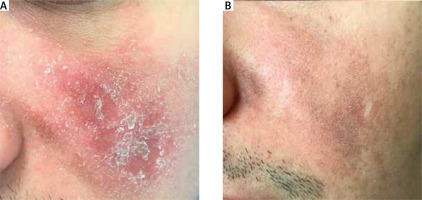
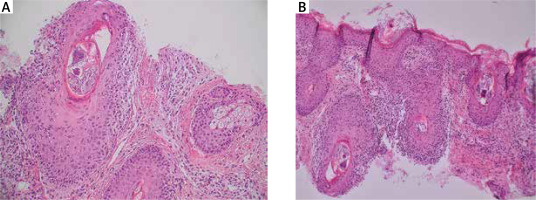
Figure 2
A, B – The histopathological examination (HE staining) with numerous structures of Demodex mites within the dilated hair follicles and reactive lymphohistiocytic infiltrates around the hair follicles, under the epidermis and around the blood vessels
Biological agents targeting TNF-α have been used in the treatment of inflammatory bowel diseases for many years with a well-established, increased risk of serious and opportunistic infections in patients [4, 5]. It is believed that in most people Demodex is a typical commensal, but in some patients, due to the increase in the density of the skin population and the general and local reduction of the level of immunity, it can become a pathogen causing clinical lesions [3]. In our patient, treatment with oral and topical metronidazole and topical ivermectin was successful, with all lesions resolving within 2 months. Demodicosis as an opportunistic infection is still a rarely described disease, but in recent years there have been more reports on this subject in the literature. So far it has been described in organ transplant recipients, patients with HIV infection, leukaemia, diabetes, chronic renal failure, skin tumours, patients treated with topical immunosuppressants (corticosteroids and calcineurin inhibitors), epidermal growth factor inhibitors and ultraviolet phototherapy [1, 2, 6]. Our case appears to be the first report of demodicosis associated with infliximab treatment. In our opinion, demodicosis should be taken into account as a differential diagnosis of atypical inflammatory skin lesions in patients treated with biological medications.








