Introduction
When pollen are dispersed from plants, they are released into ambient air, although their mission is to fertilise the flowers of the same species, sometimes they make unscheduled detours into human noses, skin and throats. At these sites, pollen trigger allergic reactions, which is referred to as pollinosis, which many people know as hay fever or rose fever [1]. Pollen, which have the aerodynamic size of 15–40 µm, probably cannot enter the lower thoracic regions of the respiratory tract; instead, they affect the nasal or nasopharyngeal mucous membrane [2, 3] whereas the smaller ones within 5–14 µm are easily filtered by the lower airways [4] where the allergens are leaked out on hydration [5].
Pollen allergens belong to type one hypersensitivities, their proteins are immunomodulatory substances, which play crucial roles in the sensitization and/or exacerbation of allergies such as seasonal rhinitis, conjunctivitis, asthma, bronchial constriction and obstruction, pollinosis and atopic dermatitis etc. [6] In a response to an allergen, the immune system produces immunoglobulin E antibodies which attach to immune cells, these undergo degranulation and trigger the cells to release histamine and other inflammatory chemical mediators. These chemical mediators elicit symptoms of allergy including wheezing, runny nose, itching, rashes, sneezing etc. These symptoms range from mild to life-threatening symptoms and could either be localized or systemic [7].
Between 10% and 25% of the population experience symptoms of hay fever or allergic asthma due to a pollen allergen and the incidence has increased over the past two decades, the reason(s) for this increment are only hypothetical. In Europe, the prevalence of allergic rhinitis was found to be around 25% [8]. The prevalence of confirmable allergic rhinitis in adults in Europe ranged from 17% (Italy) to 28.5% (Belgium) [9]. The incidence and prevalence of allergic rhinitis has increased worldwide over the past 10 years affecting 39.2% of school children aged 13 to 14 years in Nigeria [8, 10]. The prevalence of allergic asthma varies depending on the geographical location. Asthma is a common respiratory disease in Nigeria with up to 15 million people suffering from the disease (NTS, 2016). Pollen is one of the major environmental factors which exacerbate and induce respiratory allergies, especially asthma [11]. Results of the prevalence of ‘nasal allergy’ have been published in few studies. Gourango et al. showed that application of mango protein to mice produced a change in the physical appearance and a rise in the levels of eosinophils and neutrophils by 2–5.5% and 34–77.5% respectively, relative to the normal levels. In Japan, Japanese cedar (C. japonica) pollen is a major spring aeroallergen [12] and elicits pathogenic symptoms including rhinitis and/or conjunctival congestion accompanied by pollen-specific IgE production [1]. Pollen allergies are more difficult to diagnose and treat than other allergies because they are far more numerous and antigenically variable than other allergies and are exceedingly difficult to avoid. Assessment of pollen allergenicity has become paramount in understanding the allergenic plants in our environment, this is also important in effective diagnosis and management of pollen related allergens [13].
Oreodoxa oleracea is an ornamental palm plant, which belongs to the Arecaceae family. It has become increasingly used as an indoor and outdoor plant in households, large hotels and airport terminal buildings to enhance floral aesthetics.
Aim
The aim of the present study is to evaluate the allergenic potential of Oreodoxa oleracea in mice. No previous research was carried out on Oreodoxa oleraceae allergenicity.
Material and methods
Sample collection
Bunches of mature Oreodoxa oleracea flowers with yellow colour were collected from Yaba College of Technology environs, Yaba, Lagos, Nigeria. The anthers were carefully detached from the inflorescence and dried at room temperature for 3 days indoors in plastic trays.
All chemicals and reagents used in the study were of analytical grades and procured from Asophos chemicals, Ojuelegba, Lagos, Nigeria. Immunoglobulin E (IgE) kit GWB 626057 used for the measurement of the antibodies was procured from GenWay Laboratory Technologies, United States of America.
Extraction of pollen protein
Anthers were defatted with 50 ml of diethyl ether three times. They were homogenized with mortar and pestle in 100 ml of 0.02 M phosphate buffered saline (PBS) at pH 7.4. The mixtures were stirred overnight at 4°C, filtered with a muslin cloth, centrifuged and supernatant retained. The protein precipitation was carried out using 50 g of ammonium sulphate and dialysed against PBS overnight. Protein concentration was assayed according to Bradford procedures. The extract was concentrated by freeze-drying and the pollen proteins were stored at –80°C for later use in inoculating into experimental animals (mice).
Inoculation of the pollen protein extract
Male albino mice which were 4–6 weeks old were purchased from the Nigerian Institute of Medical Research, maintained under a 12-hour light-dark cycle with free access to water and standard laboratory food. All experimental procedures conformed to international standards of animal welfare and an ethical approval was obtained from the Nigerian Institute of Medical Research. Protein extracts were reconstituted with distilled water, mice were inoculated with 100 mg/kg of the pollen protein by two subcutaneous and one intranasal injections weekly for 4 weeks, control mice received an equivalent volume of phosphate buffered saline. Blood samples were obtained by retro-orbital bleeding using heparinized capillary tubes and both pre and post sera obtained were stored at –80°C for later use in detecting immunoglobulin levels. Thin blood smears were also obtained from the tail vein, fixed with methanol and flooded with Leishman’s stain for 7–10 min and washed in a stream of buffered water. Quantification of immune cells was based on a count of hundred immune cells per slide.
Immuno assay for determination of immunoglobulin E in mice sera
Elisa IgE kit GWB 626057 purchased from GenWay Laboratory Technologies, United States of America, was employed for the immunoassay. All manufacturer’s instructions on reagent dilution with distilled water were strictly adhered to and prepared prior to use. Test samples (100 µl) were measured into predesignated microtitre wells and incubated for 30 min. After removal of unbound protein by washing with Elisa machine, the wells were blotted to remove residual buffer. Anti-IgE antibodies conjugated with horseradish peroxidase (HRP) were added and incubated for 30 min, this was followed by another washing. The enzyme bound to the immunosorbent was assayed by the use of chromogenic substrate, 3,3’,5,5’-tetramethylbenzidine (TMB). Absorbance at 450 nm was determined which was a measure of the concentration of immunoglobulin E in the test sample. The values of immunoglobulin E in the test samples were interpolated from the standard curve constructed from the standards and corrected for sample dilution.
Histological examination
Animals were sacrificed after the 4th week by cervical dislocation and dissected. The respiratory organs were obtained and processing of tissue samples for histological assessment followed established procedures of Kuo (2007) [14]. The data obtained were analysed using the SPSS statistical package version 20 (SPSS Inc. Chicago, Illinois, USA). Descriptive and frequency statistics were generated to examine the means of immune cells and immunoglobulin E.
Results and discussion
Protein concentration and effect in mice
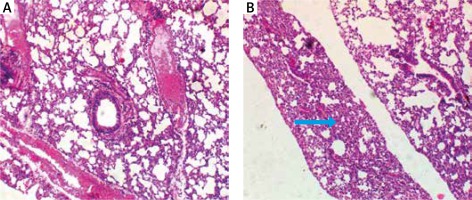
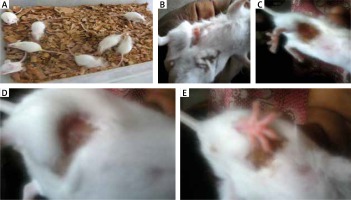
Bradford protein assay revealed a protein content of 208.11 µg/ml in Oreodoxa oleracea pollen. The pollen protein elicited allergenic extrinsic inflammatory reaction which physically presented as swelling, rashes and hair loss on the skin compared to control mice (Figure 1). Intrinsic inflammation within the lung parenchyma was also elicited (Figure 2). This agrees with Shalaby et al. who observed that allergenic proteins possess inherent properties or are associated with danger signals that target receptors, mediating allergic inflammatory responses [15]. Shahali et al. however revealed noticeable differences in protein content of two varieties of Cupressus arizonica; C. arizonica var. arizonica and C. arizonica var. glabra planted in Tehran; their work showed that there are some intraspecies specificities in their pollen protein content which may be influenced by environmental conditions [16].
Figure 1
Physical features of allergy: A – control mice showing no physical feature of allergy, B – mice inoculated with Oreodoxa oleracea showed a feature of allergy, which physically presented as swelling, C, D – mice inoculated with Oreodoxa oleracea showed a sign of allergy, which presented as hair loss, E – mice inoculated with Oreodoxa oleracea showed a sign of allergy, which presented as rashes
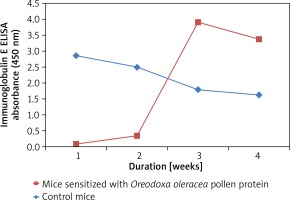
Pollen-specific immunoglobulin E (IgE) in mice sera
Mice sensitized with the Oreodoxa oleracea pollen protein showed an increase in immunoglobulin E, relative to the control mice (Figure 3), hence its pollen protein shows allergenicity in mice. Immunoglobulin E has long been known as the landmark of allergy. Babu et al. [17] also showed that immunoglobulin plays a key role in allergic reactions, particularly in the early phase response. Therefore, measurement of allergen-specific immunoglobulin E is indicated for evaluating pollen allergy. There was observable allergen sensitivity on the skin and respiratory route which were the two sites of inoculation. This finding is consistent with a study by Edimyor et al. [18], which shows that allergen exposure through the nasal and respiratory mucosa induces a strong rise of allergen-specific immunoglobulin E levels, which are responsible for increased allergen sensitivity in the target organs of allergy. Many studies have investigated the use of specific immunoglobulin E antibodies as a diagnostic tool in allergic diseases [19]. However, studies have indicated that total immunoglobulin E (IgE) value could be influenced by many factors such as gender, age and race [20, 21].
Immune cells present in mice
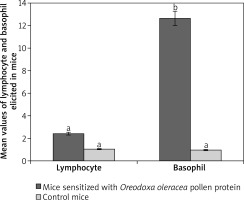
Among the immune cells only lymphocytes and basophils were abundantly produced in response to Oreodoxa oleracea pollen protein allergen.
Lymphocytes were 87% at week one before sensitization, they decreased after the second sensitization to 58% and increased to 99% after the third sensitization, on a count of less than twenty fields, indicating infiltration unlike in control mice. In control mice which received phosphate buffered saline, lymphocytes dominated other leucocytes from week 1 to week 4, but on a count of more than fifty fields. Total lymphocytes elicited by Oreodoxa oleracea were not significantly different from the controls (p < 0.05) (Figure 3). The infiltration of lymphocytes in sensitized mice correlated with an increase in IgE in mice, indicating a profound role that lymphocytes play in immunity and allergy [22].
Oreodoxa oleracea pollen protein allergen skewed basophil production at week 2 of inoculation (62%), it became lowered at week 3 (1%). Total basophils elicited by Oreodoxa oleracea were significantly different from the controls (p < 0.05) (Figure 4). The infiltration of basophils could be responsible for both extrinsic (dermatophytic reactions) and intrinsic (inflammation within the lung parenchyma) properties. Gibbs [23] noted that despite their relatively low numbers compared with other effector cells, such as mast cells and eosinophils, they have long been suspected as a major player in initiating and maintaining allergic inflammation and disorders [24].
Figure 4
Mean values of lymphocytes and basophils elicited by pollen protein and PBS in sensitized and control mice respectively. Mean bars with different alphabet letters (a, b) are significantly different (p < 0.05). Lymphocyte and basophil are expressed by mean values divided by the number of microscope fields
The clinical features of inflammation within the lung parenchyma may contribute to the development of airflow limitation by increasing airway resistance, which is a typical feature of asthma. Adrian et al. [25] showed that asthma is characterized by thickening of the airway wall and by the presence of the inflammatory process, though different among various kinds. The allergic inflammation induced by Oreodoxa oleracea could trigger not only bronchial but also lung parenchyma remodelling and these histological changes are associated with concurrent changes in respiratory mechanics of the animal.
Mortality rate
A high mortality rate was observed in mice inoculated with Oreodoxa oleracea pollen protein after the third sensitization and was not observed in control mice. The present study shows that pollen released from Oreodoxa oleracea are allergenic and could trigger allergies in hypersensitive individuals, its allergenicity was found to possess both extrinsic and intrinsic properties in mice, there is further need for its allergenicity studies in higher organisms, to fully elucidate its mechanisms especially as it is recently a priority for aesthetic purposes.